Introduction
Anastomotic leakage is the most feared complication after upper gastrointestinal surgery. Both subclinical and symptomatic cases entail an uncertain prognosis and are related with increased morbidity and mortality. The reported incidence is 2.1% to 14.6% [1–4]. The mortality rate was reported up to 50% [2, 4–7].
The traditional, standard approach to treat an anastomotic leak consists of conservative treatment with nil per mouth, abscess drainage, and surgical re-exploration with repair of the anastomosis. However, the high risk of operative morbidity and mortality is a major concern [5].
Fully covered self-expandable metal stents (FC-SEMSs) are one of the pillars of the palliative treatment of oesophageal cancer [8] and are useful for treating numerous oesophageal diseases, including anastomotic leaks, strictures, perforations, and fistulas after gastroesophageal surgeries [9, 10]. However, despite improvements in design, the migration rates of FC-SEMSs are high [9, 11].
Various methods have been used to prevent stent migration. Proximal flare can be adhered to the oesophageal mucosa by using through-the-scope (TTS) endoclips [12], over the scope clips (OTSC) [10], or endoscopic suture devices [13]. Also it can be adhered to the earlobe or nose using the shim technique [14] and by using a polypectomy snare [15].
Aim
The aim of the present study was to show that, as a different preventive method, anchoring of the distal flare of the FC-SEMS to the jejunum by using TTS endoclips is feasible and effective for stent migration.
Material and methods
From July 2020 to June 2022, we retrospectively reviewed the medical records of patients who received a FC-SEMS capable of being fixed to the jejunum by using TTS endoclips for anastomotic leak of the upper gastrointestinal tract, in the Surgical Clinic of Gastroenterology. A total of 7 patients were enrolled to the study. All procedures were performed by a single gastroenterological surgeon. Written informed consent was obtained from all patients before the procedures. The study was approved by the Local Ethics Committee (Number: 2022/6/4).
Their age, sex, body mass index (BMI), and American Society of Anesthesiologists (ASA) score were documented. Underlying causes, performed surgeries, stent indications, time interval between gastrectomy and stent insertion, stent migration, and stent extraction time were evaluated.
All data were recorded in a Microsoft Excel file. Categorical data of the patients are presented as mean ± standard deviation. Continuous variables are expressed as number and percentage.
Patient management, endoscopic procedure for stent placement, and clipping
The diagnosis of a leak was made by performing a Gastrograffin swallow and endoscopy. Abdominal and chest computed tomography were performed to evaluate the presence of abnormal fluid collection or abscess in the abdominal or thoracic cavity.
The stenting procedure was performed at the operating theatre under general propofol-induced anaesthesia, with tracheal intubation in all cases. The endoscopes used were adult gastroscopes. The upper and lower distance of the fistular orifice was determined by measuring the distance to the incisors. The stents length were 4 to 6 cm longer than the fistular orifice.
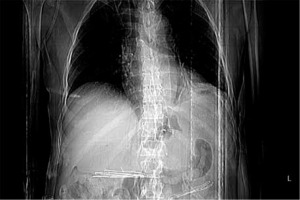
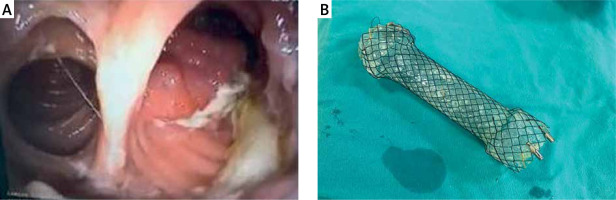
After the guidewire was inserted, the delivery system of the stent was gently introduced and the stent deployed. The positioning of the stent was assessed endoscopically by measuring the distance from the distal flare of the stent to the incisors. The lower end of the stent was passed, and a retroversion was performed in the jejunum. The parts where the lower flare of the stent did not fit into the jejunal wall were fixed to the mucosa with 1 or 2 endoclips (Figure 1). The distal mobility of the stent was controlled by applying force in the distal direction with forceps. Twenty-four to 48 h after the stenting procedure X-ray imaging or abdominal and chest computed tomography were performed to check that the stent was correctly positioned. Patients with appropriate stent localization (Figure 2) without ileus findings were fed orally with a liquid diet and enteral nutrition.
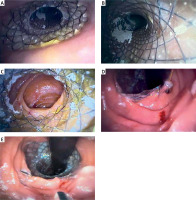
Figure 1
A – Endoscopic view after the stent deployment, a: proximal flare of the stent. B – view through the stent, C – distal flare of the stent, D – retroversion in the jejunum and the lower flare of the stent fixed to the jejunal wall with a clip, E – retroversion in the jejunum and the lower flare of the stent fixed to the jejunal wall with a second clip
All stent extractions were performed between 4 and 6 weeks after SEMS placement (Figure 3).
Results
A total of 7 patients (4 male and 3 female) underwent fully covered SEMS placement. The mean age of the patients was 59 ±13.8 years, and the mean body mass index was 29.8 ±8.4 kg/m2. The American Society of Anesthesiologists (ASA) score was ASA IV in 1 (14.3%) patient, ASA III in 3 (42.85%) patients, and ASA II in 3 (42.85%) patients. The underlying causes, performed surgeries, and stent indications are mentioned in Table I.
Table I
Demographic and clinical characteristics
The time between gastrectomy and stent insertion was 6.7 ±6.1 days. Adequate positioning and stent deployment with complete bridging of the leak was achieved in all patients. Stent migration was not observed in any of the patients during the follow-up. One of the 7 patients died due to multiple organ failure and sepsis on postoperative day 5. Except for this case, all stents were removed between 4 and 6 weeks after placement – the mean time was 37 ±4.6 days. Complete resolution of the leak was achieved in 6 patients. Stent-related perforation, stent migration, and bleeding needing endoscopic intervention or transfusion were not observed (Table II).
Table II
Properties of the applied stents and outcomes
Discussion
Postoperative anastomotic leak after upper gastrointestinal surgery is a serious concern. The mortality rate is reported to be as much as 50% [2, 4–7]. The traditional standard approach to treat an anastomotic leak consists of abscess drainage and surgical re-exploration with repair of the anastomosis. The high mortality rate of reoperation [5] and, compared with surgical intervention, the FC-SEMS placement’s minimal invasiveness, shorter procedure time, the possibility of early oral intake within 1 to 3 days, and the advantage of shorter hospitalization time [16], have made endoscopic stent deployment popular.
When the FC-SEMS is deployed, the nitinol component progressively recovers to its original shape, which results in maximal radial compression force in a period of 24 to 48 h [17]. During this time an increase in repeated oesophageal contractions with a decrease in lower oesophageal sphincter pressure [18, 19] may result stent migration. This requires repeat stenting, makes the procedure less cost effective, and may result ulcers, bleeding, and perforation.
Several methods for preventing stent migration have been reported. Proximal flare can be adhered to the mucosa by using a TTS endoclip [12], over-the-scope clip (OTSC) [10], or endoscopic suture device [13]. Also, it can be adhered to the earlobe or nose with the shim technique [14] and by using a polypectomy snare [15].
A silk thread attached at the proximal end of the stent, fixed to the nose or ear lobe through the nose is called the shim technique [14]. However, this fixation has not received wide attention to date, and such stents are not easily available. Fixation of the oesophageal stent using a polypectomy snare and affixing it to the patient’s earlobe [15] induces nasopharyngeal discomfort and requires hospital surveillance. Although data regarding OTS clipping devices [10] and endoscopic full-thickness suturing [13] are promising, their use can increase procedural costs by ~$300–$800 [20].
So, what fixation technique should be used? Any method used to anchor a fully covered stent should be easy to handle, removable, and cost effective. TTS endoclips, the necessary material for our technique, are widely available in all interventional digestive endoscopic suites. A learning curve is not needed, and the cost is much lower.
The greater distensibility and mobility of the jejunum may facilitate migration of the stent, allowing retroversion with the endoscope. Also, the permanent infoldings of the mucous membrane, the plicae circulares, enable fixation of the stent by using TTS endoclips. Hence, we used TTS endoclips unconventionally for fixation of the distal flare to the jejunum instead of fixation of the proximal flare to the oesophagus. Adequate positioning and stent deployment with complete bridging of the leak was achieved in all patients. Stent migration was not observed in any of the patients during the follow-up.
There are some limitations of this study. First, the present study was retrospective in design. Second, the small sample size of our study may not allow for generalization of the study results.
Conclusions
Our study shows that anchoring of the distal flare of the fully covered SEMS with TTS endoclips is feasible, and it may reduce the risk of stent migration. This inexpensive and safe technique may be proposed to patients with factors predictive of FC-SEMS migration. These results will of course have to be confirmed in the future by prospective randomized studies.