INTRODUCTION
Hereditary angioedema (HAE) is a rare inherited autosomal dominant disease caused by mutation in the SERPING1 gene encoding the C1 inhibitor (C1INH). Symptoms include self-limiting angioedema of the subcutaneous or submucosal tissue (particularly in the gastrointestinal or respiratory tract), resistant to treatment with glucocorticoids or antihistamine drugs [1–3]. Angioedema attacks affect various parts of the body, have different levels of severity, last from 2 to 5 days, and resolve spontaneously or with treatment. They manifest as subcutaneous swelling typically of the hands, feet, and face as well as submucosal edema of the gastrointestinal tract and, less often, of the larynx and pharynx [4].
Angioedema of the abdomen, typically affecting the gastrointestinal tract, constitutes a significant challenge. Symptoms include acute abdominal pain, severe bloating, vomiting, and diarrhea. Abdominal edema is a common manifestation of HAE, occurring in 43% to 93% of patients [4–8]. In many cases, abdominal attacks are the first manifestation of the disease, while in some patients, they remain the only symptom for years [7, 9, 10]. They constitute a significant diagnostic challenge, especially at emergency and surgical departments, because each presenting patient has to be tested for other possible causes of acute abdominal pain [11, 12]. The insufficient knowledge on HAE and its diagnosis leads to poor decision making about the treatment of the disease and its complications [5–7, 10]. It may also lead to unnecessary diagnostic laparotomy [5, 6, 10] as well as a long delay in diagnosis [5, 6, 10].
AIM
We present the case of a patient with C1INH-HAE manifesting with an abdominal attack, in whom the course of treatment was complicated by perforation of the previously undiagnosed chronic peptic ulcer.
CASE REPORT
A 30-year-old man was admitted to the emergency department due to recurrent severe abdominal pain lasting for 3 h. Pain occurred at various sites, but predominately in the upper abdomen. It was accompanied by severe bloating, weakness, and nausea but without vomiting and diarrhea.
Medical history revealed that the patient has experienced similar abdominal attacks since the age of 18. The episodes lasted for 2 to 3 days and spontaneously resolved. They occurred several times a year but for a year their frequency has increased to 2–3 attacks a month. In addition, the patient suffered once a month from attacks of subcutaneous edema of the hand, feet, face resolving spontaneously after 2–3 days. Once a year the patient had laryngeal edema. The first manifestation of the disease was severe hand swelling after a finger injury at the age of 17. Family history was positive. The mother as well as 3 brothers and a sister suffered from similar symptoms.
At 18 years of age a deficiency of C1INH was confirmed in the patient (Table 1) and hereditary angioedema type I due to C1 inhibitor deficiency was diagnosed. The patient was treated symptomatically. He was given Danazol on subcutaneous edema.
TABLE 1
Laboratory test results
Abdominal attacks as well as angioedema of the larynx or face as recommended were successfully treated with intravenous plasma-derived C1INH concentrate (pdC1INH) (Berinert; CSL Behring) or sc icatibant (30 mg). Symptoms of edema attacks usually resolved within 2–3 h [1–3].
Physical examination of the patient on the day of admission to the emergency department showed signs of dehydration (dry skin and mouth), reduced blood pressure (100/70 mm Hg), and increased abdominal girth were observed. Surgical assessment revealed severe bloating, generalized abdominal tenderness, no peritoneal symptoms, and normal intestinal motility. There were no indications for surgical treatment. Laboratory tests (Table 1) indicated a slight increase in C-reactive protein levels. The diagnosis of an abdominal attack in the course of HAE was established. The patient was treated with intravenous pdC1INH (20 U/kg), fluid therapy (2.5 l), and antispasmodic drugs. He was discharged after 3 h in a general good condition with the recommendation to use the antispasmodic drug as needed. He was also instructed to present to the emergency department if the symptoms recurred.
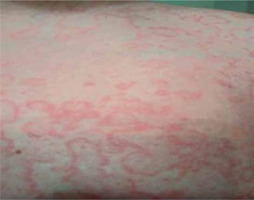
Moderate abdominal pain, mainly in the upper abdomen, recurred after several hours since discharge. The patient took 10 mg hyoscine butylbromide as recommended. After another hour, he developed generalized erythema with severe burning sensation without urticaria (Figure 1).
The patient was admitted to the emergency department again, where an allergic reaction to the drug taken was diagnosed. Physical examination of the abdomen revealed slight abdominal tenderness, no peritoneal symptoms and normal intestinal motility. There was still no indication for surgical treatment. The patient received antazoline (100 mg im) and dexamethasone (8 mg). He was discharged again after an hour despite recurrent mild abdominal pain and persistent erythematous lesions. He was instructed to present again to the emergency department in the case of symptom worsening. Three hours later, abdominal pain became more severe particularly in the upper abdomen. The patient was readmitted to the emergency department again.
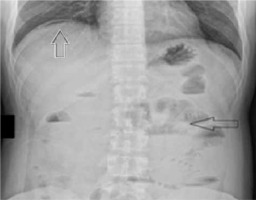
This time on physical examination, peritoneal symptoms were present. He had elevated temperature (38.5°C). Abdominal X-ray showed a pneumoperitoneum indicating gastrointestinal perforation (Figure 2). Abdominal ultrasound revealed a significant amount of free fluid, enlarged spleen, and thickening of the gallbladder wall. The patient was referred to the surgical department. Laboratory test results showed significant increase of C-reactive protein levels (202 mg/l; reference range < 5 mg/l >). White blood cell count was normal (5.7 × 109/l; reference range < 4.5–11 × 109/l >). He was administered pdC1INH 20 U/kg, following which the surgery was performed.
FIGURE 2
Abdominal X ray. White arrow indicates a pneumoperito- neum, black arrow indicates signs of subileus
Laparotomy revealed perforation of the chronic duodenal ulcer. During the surgery, a significant amount of free peritoneal fluid with pus and traces of bile was revealed. Peritonitis was caused by perforation of the anterior wall of the pylorus. The chronic peptic ulcer with extensive necrotic tissue was cleansed and sealed, and the peritoneal cavity was thoroughly rinsed. The surgery was successful with no complications. After 7 days, the patient was discharged in a good general condition. Several days after the surgery, C-reactive protein levels gradually decreased (to 34.8 mg/l over a few days), ultrasound revealed no free fluid in the peritoneal cavity, and the spleen size was reduced.
After the surgery and during a 2-year follow-up, the patient reported only one abdominal attack and one episode of laryngeal edema, while hand, foot, and face swelling occurred with the same frequency – several times a year.
DISCUSSION
Abdominal attacks are a common manifestation of C1INH-HAE [3–5]. They constitute a considerable diagnostic and therapeutic challenge, especially at emergency, surgical, gastroenterology, and obstetrics units, where the general awareness of this rare condition is low [5, 6, 13, 14]. Greater awareness is particularly important because in each case of an abdominal attack (in severe cases, 2–3 times a month), other causes of acute abdominal pain must be excluded. The diagnosis is especially difficult when the patient has not been previously diagnosed with C1INH-HAE, when an abdominal attack is the only manifestation of the disease, or if it occurs as the first symptom of the disease. These diagnostic challenges are reflected by a high rate of diagnostic laparotomies in patients with an abdominal attack, which in most cases reveal only a significant amount of peritoneal fluid or segmental intestinal edema. However, in some cases, laparotomy is justified as it allows a diagnosis of appendicitis, cholecystitis, or ovarian cyst rupture [5–8, 10, 13, 14].
We present an untypical case of an abdominal attack in the course of C1INH-HAE complicated by perforation of the previously undiagnosed chronic peptic ulcer. Prior to the presentation, abdominal attacks resolved in the patient within 2 h from the administration of pdC1-INH or icatibant.
This time improvement of the patient’s symptoms after pdC1INH administration was only short-lasting, with recurrence after several hours. Generalized erythema was diagnosed as an allergic reaction to an analgesic, but this might have been marginal erythema, which is a common symptom in HAE attacks [2, 3, 10]. After several hours of recurrent abdominal symptoms, the patient again developed acute abdominal pain and peritoneal symptoms appeared with temperature. Laparotomy was considered necessary. It revealed perforation of the chronic peptic ulcer in the course of HAE attack, as indicated by abundant free fluid in the abdominal cavity. After the removal of the ulcer, abdominal attacks basically resolved. During the 2-year follow-up, the patient reported only one abdominal attack. It seems that the increased frequency of abdominal attacks to 2–3 a month in the year before the presentation was caused by the undiagnosed chronic peptic ulcer, which may be an inflammatory focus and inducer of HAE attacks in the patients as indicated by other authors [7, 9]. A significant improvement and resolution of abdominal attacks observed in the patient after the removal of the peptic ulcer seems to indicate the need to search for gastrointestinal abnormalities, inflammation in the case of an increase in the frequency of abdominal attacks in a patient with HAE. Treatment of any abnormalities in the digestive tract in these cases may have an important effect on reducing angioedema episodes and especially and especially abdominal attacks.
CONCLUSIONS
If symptoms of abdominal attack in a patient with C1INH deficiency do not disappear for 2–3 h after usually effective pdC1INH or icatibant administration or come back, the patient should be hospitalized and observed, have an abdominal CT and extended laboratory examinations made to exclude other causes of acute abdominal symptoms.
An increase in the frequency of abdominal attacks in a patient with C1 inhibitor deficiency requires the exclusion of inflammatory foci and other diseases in the gastrointestinal tract.