Introduction
In recent years, the incidence of gastroesophageal junction tumours has increased rapidly throughout the world [1]. Moreover, in a recent review, oesophagogastric junction cancers were reported to have a mortality rate of 85%, so screening is vital for precaution of oesophagogastric junction tumours. Also, the 5-year overall survival rate in oesophagogastric junction (OGJ) tumours ranges from 23 to 38% [2, 3].
Tumour markers are proteins, glycoproteins, or other materials produced by tumour cells and anticores exposed to these materials. Tumour markers are used in cancer screening, cancer prognosis, and disease progression. Serum CEA is a glycoprotein that plays a part in cell adhesion [4]. CA 19-9 is a glycolipid antigen and also a ligand for E-selectin. Both markers have been found in colorectal cancers [5]. Serum CEA and CA-19-9 are the most used markers, despite there is not any specific tumour markers for OGJ tumours [6].
Preoperative CEA and CA 19-9 elevation and prognosis in stomach cancer have been shown in some studies [7, 8]. A powerful serum tumour marker will help to develop the clinical management of OGJ adenocarcinoma. Nevertheless, several studies in the literature have evaluated the clinical usefulness of serum tumour markers in OGJ adenocarcinomas [1, 9, 10].
Aim
Our intention here is to depict the association between the elevation of serum CEA and CA 19-9 in clinicopathologic features and survival in OGJ adenocarcinoma.
Material and mthods
In the present, retrospectively designed study, the data of patients who were operated in the gastrointestinal surgery clinic due to OGJ adenocarcinoma were examined. Approval was granted by the Ethics Committee of the hospital in which the study was conducted (No:2019.7/40-256). At the same time, the study was carried out in line with the ethical standards of the Helsinki Declaration (revised in 2013). Patient files in the hospital archive from 2007 to April 2019 were examined. Patients aged 18 years or older, diagnosed with OGJ adenocarcinoma, and undergoing elective surgery were included. Patients who had acute pancreatitis and other cancer types were excluded because CEA and CA 19-9 are elevated in other cancer types and acute pancreatitis. Survival data of all patients were obtained, and curative surgical resection was performed in all patients. The staging of the patients was performed using oesophagogastroscopy, high-resolution thorax, and whole abdomen computed tomography (CT) and positron emission scintigraphy (PET). Also, the decision regarding neoadjuvant chemotherapy (NAC) was made by the multidisciplinary team. The staging of the patients was made according to the 7th edition of the IUCC classification. Demographic features: sex, age, medical history of the patients, smoking, body mass index (BMI), and American Society of Anaesthesiologists (ASA) score were recorded. In addition, the presence of (NAC), lymphovascular invasion (LVI), perineural invasion (PNI), positive lymph node number (N +), tumour differentiation, and tumour diameter were recorded. OGJ adenocarcinomas were classified according to Siewert classification [11]. Surgical resection was performed according to the 4th edition of the Japanese Guidelines [12].
Serum CEA and CA 19-9 were examined at diagnosis, and cut-off values were determined as 5 ng/ml for serum CEA and 37 U/ml for serum CA 19-9 in view of previous studies [1, 13]. Patients were followed up until death or until May 2020, whichever occurred first.
Statistical analysis
SPSS (Statistical Package for the Social Sciences) 23.0 was performed for statistical analysis of the data. Categorical variables were briefed as numbers and percentages, and mean and standard deviation (median and minimum-maximum where necessary) in continuous variables. Pearson’s χ2 test statistics were performed to compare categorical variables. Shapiro-Wilk test was applied to depict whether the parameters in the study demonstrate normal dispersion. In comparing the continuous measurements between the groups, the distributions were checked and the independent Student’s t-test was performed for the parameters with normal dispersion, and Mann-Whitney U test for the parameters without normal dispersion. Kaplan-Meier analysis and Log Rank tests were performed in survival analyses. The logistic regression test was applied to determine the independent variables affecting survival. The statistical significance level was assumed at 0.05 in all tests.
Results
The mean age of the 70 patients who took part in the study was 59.78 ±10.49 (31–76) years. Fifty-three of the 70 patients were male and 17 were female. Thirty-eight (54.3%) of those diagnosed with OGJ adenocarcinoma were suitable for Siewert II and 32 (45.7%) for Siewert III classification. The median serum CEA was 2.8 ng/ml (0–313), and CA 19-9 was 12.5 U/ml (0.8–1700). Serum CEA and CA were 19-9 high in 19 (27.1%) of 70 patients. In our study, the value range with CEA > 5 was between 6 and 313 and the CA 19-9 > 37 value range was between 40 and 1028. Both tumour markers were high in 8 (11.4%) patients. The mean diameter of the tumour was 4.89 ±2.41 (0.8–15) cm. The clinical stage of most patients was stage II and stage III (90%). The follow-up period of the patients ranged from 1 to 110 months, with a mean 30.6 months. Along the follow-up period, 31 (44.2%) patients were observed to die due to cancer-related reasons.
The relationship between clinicopathological properties and serum CEA and CA 19-9 positivity (≥ 5, ≥ 37, respectively) is shown in Table I. The patients were grouped in two ways as < 5 (negative) and ≥ 5 (positive), according to serum CEA value. As a result of the examination, the age, sex, tumour depth (T), clinical stage, tumour differentiation, LVI, PNI, tumour type, presence of smoking, diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), perioperative immunonutrition, coronary artery disease (CAD), ASA score, survival, age, N+, tumour diameter, BMI values, and differences between CEA groups were not statistically significant (p > 0.05).
Table I
The association between clinicopathological characteristics of patients and serum CEA and CA 19-9
CEA ≥ 5 ng/ml was found to be statistically significant in patients receiving NAC and in patients with N+ (p = 0.041, p = 0.042, respectively). In the study, the parameters of the patients were examined in two groups as serum CA 19-9 value < 37 (negative) and ≥ 37 (positive). As a result of the examination, patients’ sex, tumour depth (T), clinical stage, tumour differentiation, PNI, tumour type, smoking presence, COPD, perioperative immunonutrition, CAD, ASA score, N+, tumour diameter and BMI values and differences between CA 19-9 groups were not statistically significant (p > 0.05). The CA 19-9 positivity rate was found to be statistically significantly higher in patients aged 60 years and older (p = 0.001).
CA 19-9 positivity was statistically higher in patients with LVI and diabetes DM (p = 0.042 and p = 0.012, respectively). The mortality rate was statistically slightly higher in the group with negative CA 19-9 (p = 0.048). The age and N+ findings of the patients in the CA 19-9-positive group were statistically significant compared to the patients in the CA 19-9-negative group (p = 0.039 and p = 0.007, respectively).
The estimated mean survival rates of the patients in this study are given in Table II. Accordingly, while the overall survival was determined as 51.07 ±6.89 months, the 1 year survival rate was 77.4%, 3-year survival rate was 48.5%, and 5-year survival rate was 35.4%. In Table III, comparisons of patients with overall survival rate and survival rates between CEA groups are given. The differences between the two groups were not statistically meaningful.
Table II
The mean overall survival time of OGJ adenocarcinomas
| Parameter | Mean | 1-year survival (%) | 3-year survival (%) | 5-year survival (%) | |||
|---|---|---|---|---|---|---|---|
| Mean Predictive | SD | 95% confidence interval (CI) | |||||
| Lower limit | Upper limit | ||||||
| Survival | 51.07 | 6.89 | 37.55 | 64.59 | 77.4 | 48.5 | 35.4 |
Table III
Overall survival rates of patients and survival rates between CEA groups
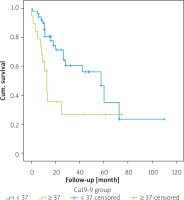
The 1-year survival rate of those who were CA 19-9 negative was 80.5%, the 3-year survival rate was 56.1%, and the 5-year survival rate was 35.1% (Table IV). The 1-year survival rate of patients who were CA 19-9 positive was 49.7%, 3-year survival was 26.6%, and the 5-year survival rate was 26.6%. Overall survival rates of 1–3 and 5 years were statistically significantly lower in patients who were CA 19-9 positive (p = 0.016) (Figure 1).
Table IV
The association between survival and CA 19-9
In multivariate logistic regression analysis, we aimed to examine the independent risk factors affecting the study group by taking the survival effect of the patients as the dependent variable. As a result of the logistic regression analysis, the parameters of tumour diameter (OR = 0.605), LVI presence (OR = 0.280), and PNI presence (OR = 0.123) were shown to be an independent risk factor (p = 0.001, p = 0.022, p = 0.009, respectively) (Table V).
Table V
Multivariate analysis of the relationship between patients’ characteristics and overall survival
In Table VI, when both tumour markers were positive and only one tumour marker was positive, patient characteristics were compared, and the rate of CA 19-9 positivity was found to be statistically significantly slightly elevated in women only (p = 0.011). The ratio of DM (p = 0.046) and CAD presence (p = 0.032) was statistically significantly higher in patients with both positive tumour markers. In patients who had both tumour markers positive, the N+ mean value was statistically significantly higher (p = 0.001).
Table VI
Background characteristics in the groups who were double positive and single positive for CEA and CA 19-9
Discussion
Many studies with large series have examined pretreatment serum CEA and CA 19-9 to estimate the prognosis of stomach cancer. Also, several studies have examined the relation of these tumour markers with prognosis and clinicopathology of OGJ adeno cancers. In these studies, OGJ adenocarcinomas and OGJ squamous cell cancers (SCC) were generally evaluated together [1, 10, 13, 14].
In our study, the relationship between preoperative serum CEA and CA 19-9 positivity and overall survival in patients who underwent only curative resection diagnosed with OGJ adenocarcinoma was examined, as well as the relationship between the patients’ clinicopathological features and tumour markers. As a result of clinicopathological examinations, a significant relationship was shown between the number of patients receiving NAC and the elevation of CEA and N+, while a statistically significant difference was seen between CA 19-9 positivity in patients over 60 years of age. In addition, the ratio of LVI and DM was statistically higher in those with a high CA 19-9 value. Again, those with CA 19-9 elevation were older and the N+ ratio was higher. Unlike our study, in a study involving 1075 series gastric cancer cases, a significant association was found between CEA and LVI [15].
Scarpa et al. [9] reported that the incidence of increased serum CA 19-9 in OGJ cancers was 12.3%, while in our study this rate was 27.1% for both tumour markers and was similar to that of another study (22.4%) [15].
Similar to the 211 series two-centre retrospective study, it was found in the present study that the CA 19-9-positive group had 1-3- and 5-year overall survival that was significantly lower than the -negative group [1]. Conversly, the mortality rate of the group with a negative CA 19-9 was higher than that of the positive group. On the other hand, in the same study, they did not investigate deeply the relationship between serum CEA and CA 19-9 with the clinicopathological features of the patients.
In our study, when the risk factors affecting overall survival were investigated, we revealed that tumour diameter, LVI, and PNI presence were independent risk factors affecting survival. However, we depicted that serum CEA is not an independent risk factor affecting survival.
On the other hand, in this study, we revealed that there is a significant association with CA19-9 positivity and female gender. In the study conducted by Wada et al. [6] it was demonstrated that there was a significant relationship between CEA positivity and male gender. In addition, in our study, the presence of DM and CAD was statistically higher in the group in which both tumour markers were positive. Also, a positive correlation was resulted between N+ and the rate of both tumour markers being positive.
In the present study, although no statistically significant association was found between CEA positivity and survival, the mean survival rates and 5-year survival rates of CEA-positive patients were lower than those of CEA-negative patients. A large meta-analysis highlighted that high CEA before treatment was associated with poor prognosis and almost twice the mortality [7]. Similarly, a meta-analysis involving 11,408 gastric cancers reported that high CA 19-9 was associated with poor prognosis [16]. In a study examining the serum CEA and CA 19-9 in stomach cancer in our country, an association with the elevation of these tumour markers and the stage of gastric cancer was found [17]. Although the elevation of CEA and CA 19-9 was higher in stage III OGJ adenocarcinoma, no statistical significance was found in the present study. Chen et al. depicted that serum tumour markers used to predict OGJ adenocarcinoma in OGJ malignancies were examined and showed that high CEA, CA 19-9, and CA 72-4 are associated with OGJ adenocarcinoma rather than OGJ SCC [18]. In a recent study, it was reported that high CEA and CA 19-9 before treatment in OGJ adenocarcinoma related to treatment failure and decreased overall survival [19].
Several limitations to this study should be noted. First of all, our study was designed retrospectively, and it was a small-scale and single-centre study. In addition, the data of patients included in the Siewert I classification were not available in this study. Moreover, the data of cancer-specific survival and disease-free survival times were not available. Prospective, randomised, multicentre studies are needed to examine the effects of serum CEA and CA 19-9 in OGJ adenocarcinoma on prognosis and disease-free survival.
Conclusions
A significant association was found between serum CA 19-9 positivity and OGJ adenocarcinoma. Moreover, in patients with both tumour markers positive, the N+ mean value was higher. At the same time, we have shown that tumour diameter, LVI, and PNI are independent risk factors that affect survival in OGJ adenocarcinoma.