Introduction
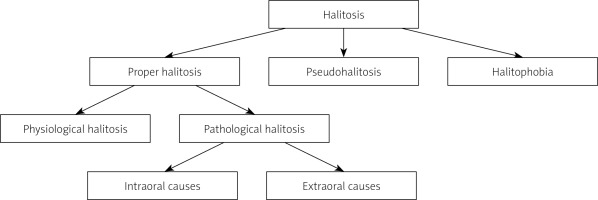
Halitosis (fetor ex ore) is defined as an unpleasant odour from the mouth, regardless of the cause: local or systemic. This is due to the presence of chemical compounds contained in the exhaled air, mainly volatile sulphur compounds (VSCs) in oral pathologies, and volatile organic compounds (VOCs) in the majority of extraoral causes [1, 2]. Murata et al. distinguish proper halitosis (physiological and pathological), pseudohalitosis, and halitophobia. In 2010, Tangerman et al. proposed a division of halitosis into intra- and extraoral forms, which were further divided into haematogenous and not related to the circulatory system.
Recently, a new aetiological classification has been proposed dividing pathological halitosis into type 1 (oral), type 2 (airway-related), type 3 (gastroesophageal), type 4 (haematogenous), and type 5 (subjective). Type 0 is considered physiological halitosis [3]. Despite newer classifications, the division introduced by Murata et al. is most commonly used (Figure 1) [4].
Classification of halitosis according to Murata et al.
Pseudohalitosis and halitophobia are false halitosis. In pseudohalitosis, the patient complains of an unpleasant odour from the mouth, which is not perceptible by others. Halitophobia occurs when a person previously treated for proper halitosis is convinced that the symptoms persist, despite the lack of evidence for the disease.
The physiological form of proper halitosis is characterized by the production of VSCs in the healthy mouth. The symptom occurs immediately after waking up (the so-called morning halitosis) [2] and is caused by reduced salivation in people breathing through the mouth or the presence of food residues.
Disturbed physiological flora as well as the appearance and growth of proteolytically active Gram-negative anaerobes (e.g. Treponema denticola, Prevotella melaninogenica, Porphyromonas gingivalis, Prevotella intermedius, Tannerella forsythia, and Fusobacterium nucleatum) lead to the production of volatile sulphur compounds, such as methyl mercaptan (CH3SH), hydrogen sulphide (H2S), and dimethyl sulphide (CH3)2S [4–8]. This is due to the ability to metabolize sulphur-containing amino acids (mainly cysteine and methionine). Increased production of VSCs by bacteria living in the oral cavity and bacteria that appear in pathological conditions and their accumulation lead to pathological halitosis associated with intraoral disorders (80–90% of cases). The main causes of the condition include the following: poor oral hygiene, untreated caries, pulp gangrene, gingivitis and periodontitis, purulent inflammation, and pathologically dry mouth (xerostomia). If an increase in the amount of VSCs is accompanied by the appearance of VOCs, pathological halitosis develops due to extraoral causes (10–20% of cases) [2]. The most common disorders include the following:
ENT: chronic sinusitis, purulent tonsillitis, deviated septum, neoplastic changes,
gastroenterological: oesophageal diverticula, severe form of gastroesophageal reflux disease, H. pylori infection,
pulmonary: bronchitis, bronchiectasis, lung cancer,
metabolic diseases: diabetic ketoacidosis, uraemia.
According to various sources, proper halitosis affects from a few to over 50% of the population [2, 9]. The correlation with gender has not been clearly determined, but the economic and social status of patients is taken into account. According to most researchers, the incidence of bad breath increases with age, which is caused, for instance, by reduced salivation, incorrect use of dentures, periodontitis, and systemic diseases. In the younger population, poor oral hygiene and caries predominate [10].
Pathological halitosis in selected gastroenterological diseases
Bad breath is not, as previously thought, a rare manifestation of gastroenterological diseases. For this reason, after excluding dental and otolaryngological causes, determination of the aetiology of halitosis should focus on the diagnosis of gastrointestinal diseases [11]. In this case, bad breath may be caused by VSCs, mainly in disorders of the upper gastrointestinal tract, and/or by VOCs.
H. pylori infection is one of the most common chronic infections [12]. The bacterium is a Gram-negative organism inhabiting mainly the mucosa of the pre-pyloric part of the stomach and duodenum, less often the lining of the oesophagus. The bacteria may temporarily appear in the mouth of patients with coexisting gastroesophageal reflux disease. Research has shown that H. pylori produces volatile organic compounds and 2 major sulphur compounds responsible for the development of halitosis: hydrogen sulphide and methyl mercaptan [11]. Serin et al. observed a significant reduction in the severity of halitosis in patients who underwent treatment due to a positive result of the H. pylori test [13]. Initially, bad breath was found in 61.5% of the subjects. After successful eradication, the percentage dropped to 12.8%. The results of a study conducted by Katsinelos et al. show an even greater reduction of the symptom from 100% to 11.2%.
Bad breath is also associated with gastroesophageal reflux disease (GERD). Currently, the GERD classification includes oesophageal and extra-oesophageal syndromes, with halitosis as one of the manifestations. Moshkowitz et al. examined 132 patients: 72 (55%) with gastroesophageal reflux, 52 (39%) with functional dyspepsia, 7 with peptic ulcer, and 1 with gastric cancer. According to the results of the research, halitosis coexisted with typical oesophageal symptoms of GERD, such as heartburn, regurgitation, and sour taste in the mouth. There was no correlation with epigastric pain, flatulence, early satiety, and chest pain. No significant relationship was found between halitosis and functional dyspepsia [14].
Retention of gastric contents observed in oesophageal diverticula, achalasia, pyloric stenosis, oesophageal cancer and gastric cancer stimulate the production of volatile sulphur compounds by Gramm-negative anaerobic bacteria. In patients over the age of 70 years with Zenker’s diverticulum, halitosis may be associated with the presence of food residues due to the underlying disease and other intraoral factors (periodontal diseases, xerostomia, improper hygiene of prosthetic restorations) [15].
Inflammatory bowel diseases, such as ulcerative colitis (UC) and Crohn’s disease (CD), are autoimmune disorders giving mainly intestinal symptoms; however, they can also cause extraintestinal problems, including those related to the oral cavity. Katz et al. [16] examined a group of 94 people, 54 of whom had IBD (34 with CD and 20 with UC). The control group consisted of 40 people. The results of the research confirmed a higher incidence of halitosis in IBD patients. 50% of the subjects with UC and 29% with CD had bad breath, compared to 10% for the control group. Additionally, patients with active IBD forms showed an increased incidence of halitosis compared to patients in remission. Increased production of volatile sulphur compounds in IBD is associated with the presence of lesions in the mouth. These include aphthous ulcers, diffuse erythematous gingival hyperplasia, acute periodontitis, and xerostomia [11]. These factors cause the multiplication of pathogenic bacteria and the production of VSCs. In IBD, we also observe a change in intestinal permeability caused by inflammation, with the presence of VOCs in the exhaled air [17, 18]. The exact mechanisms of VOC production are the subject of ongoing research. It is presumed that the process may be caused by increased oxidative stress [2] or direct fermentation of products in the diet.
The term foetor hepaticus (hepatic stink) can be found in the literature. This is a sweetish, slightly faecal, musty smell. It occurs as a late symptom of diseases leading to liver failure caused, for instance, by toxins (including alcohol), viral hepatitis, and autoimmune diseases (AIH, PBC, PSC). VSCs, in particular, methyl mercaptan, and nitrogenous substances (including ammonia), and volatile organic compounds (acetone, 2-butanone and isopropyl alcohol) are responsible for bad breath [19, 20] (Table I). Because of the phenomenon of portal hypertension and collateral circulation, substances causing halitosis can enter the systemic circulation through the connection between the inferior vena cava and the portal vein bypassing the liver, and directly enter the lungs from which they diffuse [15].
Table I
Examples of volatile organic compounds (VOCs) in diseases of the gastrointestinal tract and liver [19, 20]
The assessment of only VSCs does not allow us to distinguish between the intraoral and systemic causes of the symptom. Particular attention should be paid to VOCs.
There are 2 ways of development of halitosis as a symptom of gastroenterological origin [2]. The first is the transoesophageal release of VOCs into the mouth through the upper gastric sphincter, and the second is the adsorption of VOCs into the bloodstream with their diffusion through the lungs [21].
Analysis of the content of organic chemical compounds in the air exhaled by patients suffering from a certain disease allows assignment of a given substance to a given pathology.
Examples of VOCs in diseases of the gastrointestinal tract and liver
Studies have shown that the evaluation of a single chemical compound has no diagnostic value. A group of substances whose increase in the exhaled air is characteristic of a given disease should be taken into account.
The organoleptic evaluation of bad breath based on the 6-point Rosenberg scale is the basic method for diagnosing halitosis. An imperceptible odour corresponds to zero, while 5 points indicate the presence of bad breath. The result largely depends on the experience of the examiner; therefore, it is a very subjective method. However, there are also objective methods for assessing halitosis, including gas chromatography, portable VSC analysers, and Artificially Intelligent Olfactory (AIO).
The Halimeter (Interscan Corporation) and OralChroma (Abimedical Corporation) are used to quantify the total level of volatile sulphur compounds in the exhaled air. The results are given in ppb units (parts per billion) [22], which define the number of molecules of a chemical compound per 1 billion solvent molecules. OralChroma assesses the presence of the 3 most common VSCs: H2S, CH3SH, and (CH3)2S, while Halimeter covers all volatile sulphur compounds.
Gas chromatography along with AIO allow for qualitative and quantitative analysis of both VSCs and non-sulphur volatile organic compounds. Due to the high cost and the need to employ qualified personnel, gas chromatography is not used in everyday practice [15].
Intelligent “artificial olfactory” (AIO) is the newest method of assessing bad breath. There are products available on the market from Agilent technologies (model 4440B), Alpha Mos Prometheus, and Cyrano Sciences (Model A320). The devices include sets of sensors based on nanomaterials for detection of gases coupled with artificial intelligence. This allows for multi-directional analysis of the exhaled air, including detailed determination of both volatile sulphur compounds and volatile organic compounds. Sensor subsets process information about each detected substance individually. Then, the compounds are grouped and compared with previously developed and programmed patterns of halitosis causes. This makes it possible to determine the origin of bad breath. The method is particularly important in the diagnosis of the systemic origin, including gastroenterological diseases.
Summary
Nowadays, halitosis is becoming a growing social problem, which causes discomfort in interpersonal relationships. It should not be underestimated, because it may be a symptom of serious systemic diseases. Diagnostics and treatment of the pathology are based on close cooperation between doctors of various specialties, including dentists, otolaryngologists, and gastroenterologists. Thanks to modern technology, it is possible to perform a quick and detailed analysis of the composition of exhaled air in patients reporting bad breath. The elimination of halitosis as a symptom of gastroenterological diseases is based on the treatment of the underlying disease. Additionally, the patient should pay attention to appropriate oral hygiene, diet, and the use of odour-masking preparations [23].