Introduction
Globally Corona virus disease 2019 (COVID-19) has caused almost 2.9 million deaths, and the numbers continue to increase [1]. The therapeutic options remain limited. Few studies have reported improved clinical outcomes in hospitalized COVID-19 patients with use of famotidine [2, 3]. A case series demonstrated improved outpatient outcomes with use of high-dose famotidine [4]. Another observational study showed that combination of antihistamines including famotidine and cetirizine improved the mortality and symptom progression [5]. A recent large multicentre observational study did not show any mortality benefit with use of famotidine [6]. A recent meta-analysis by Kamal et al. showed that conclusions regarding the safety and effectiveness of PPI and famotidine cannot be drawn based on conflicting and heterogeneous data [7]. Great enthusiasm can be observed regarding the use of famotidine or H2 receptor blockers, and sometimes these agents are preferred over proton pump inhibitors (PPI), which are more affective agents for upper gastrointestinal symptoms [8]. The activation of mast cells and pathological release of histamine in the setting of COVID-19 infection leads to severe inflammation. It is thought that H2 receptor antagonist (H2RA) may attenuate this effect [9]. Also famotidine is a potential inhibitor of 3-chymotrypsin-like protease (3CLpro), which is involved in the virus replication [10]. The commonly used famotidine formulation (Pepcid Complete) contains calcium, and hypocalcaemia is commonly seen in severe COVID-19 infection. The calcium can interact with fats and impede the lipotoxicity and as a result improve outcomes [11]. Most of the available studies utilized the medication data obtained during hospitalization. The literature regarding the impact of H2 receptor blocker for long-term use or as home medication is scarce [12].
Aim
We aim to study H2 receptor antagonist (H2RA) use in hospitalized patients and its impact on the mortality and hospital-related outcomes. We also compared the outcomes in patients on H2RA with patients using PPI.
Material and methods
Study population
This retrospective cohort study was conducted at a tertiary care hospital in Albany, located in upstate New York, which has large catchment area. After a chart review, we identified all hospitalized patients diagnosed with COVID-19 infection between 1 March 2020 and 31 July 2020. The study was approved by the institutional review board of Albany Medical Centre with patient consent waived.
After chart review, we included patients above 18 years old who were diagnosed with COVID-19. Patients were excluded if they were older than 90 years. The cases with incomplete data were excluded. The baseline date was chosen from the date of hospital admission.
Baseline data and covariates
COVID-19 was diagnosed by nasopharyngeal polymerase chain reaction. Severe COVID-19 infection was defined as SpO2 < 94% in room air, ratio of partial pressure of oxygen and inspired air fraction (PaO2/FiO2) < 300 mm Hg, more than 50% involvement of lungs, and respiratory rate > 30 breaths/min. Patients with critical illness, who had multiorgan failure were also considered as severe COVID-19 [13]. Chronic kidney disease (CKD) was defined as GFR < 60 ml/min/1.73 m2 measured on 2 separate occasions [14].
Baseline demographic data including age, sex, race, and comorbidities including obesity, hypertension, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), history of cancer, liver disease, immunodeficiency, and cigarette smoking were extracted after a careful chart review. Medication use including PPI and H2RA were recorded from the medication history at time of the diagnosis or most recent prescription record before the hospital admission.
The study subjects were segregated into H2RA users and non-users. The patients on PPI were also compared with patients on H2RA.
Outcomes
The primary outcome was the mortality in the groups of H2RA users, H2RA non-users, and PPI users. The secondary outcomes included development of severe COVID-19 infection with acute respiratory distress syndrome (ARDS) and thromboembolism requiring ICU admission and mechanical ventilation. We also recorded the length of admission and the readmission in all groups.
Statistical analysis
The baseline characteristics were presented as percentages for the categorical variables and mean ± SD for continuous variables and were compared between H2RA users and non-users. A multivariate logistic regression model was used to assess the association between H2RA use and other covariates with mortality during the study period. These models were adjusted for age, sex, race, diabetes, CAD, COPD, asthma, history of cancer, chronic kidney disease, liver disease, obesity, and cigarette smoking. We also evaluated the same predictors among H2RA and PPI subgroups. All analyses were conducted using STATA 16. A p-value < 0.05 was considered significant.
Results
Demographics
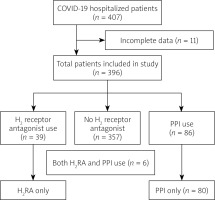
During the study period 407 adult patients were admitted from March till July 2020 with COVID-19 infection. Of those, 11 patients had incomplete data. Of the remaining 396 patients, 86 received PPI, 39 received H2RA, and 6 patients received both PPI and H2RA. Among H2RA users, 33 were taking famotidine, 5 were on ranitidine, and 1 was taking cimetidine. These 6 patients were excluded while comparing H2RA and PPI (Figure 1).
Figure 1
Flowchart showing the coronavirus disease 19 patient population
H2RA – H2 receptor antagonist, PPI – proton pump inhibitor
The demographics were similar for H2RA users and non-users. The mean age was 57.79 ±17.36 years, 43.2% were female, and 48.7% were Caucasian. The most common secondary diagnoses were hypertension (56.8%), obesity (44.4%), diabetes mellitus (38.6%), coronary artery disease (30.1%), chronic kidney disease (19.2%), asthma (15.2%), and chronic obstructive pulmonary disease (13.6%). As compared to H2RA non-users, the H2RA user group had higher percentages of CAD (19.6% vs. 38.5%, p = 0.006), asthma (13.7% vs. 28.2%, p = 0.02), and hypertension (55.2% vs. 71.8%, p = 0.05). The patients taking PPI had similar demographics and covariates as compared to H2RA users. The past and present cigarette smoking was more prevalent in patients taking PPI (42.5% vs. 18.2%, p = 0.03). Gastrointestinal symptoms were seen in 31.1%, and 43.9% of patients had abnormal liver enzymes on initial presentation (Table I).
Table I
Baseline characteristics of COVID-19 patients taking either proton pump inhibitor or histamine H2 receptor antagonist
Mortality and adverse outcomes
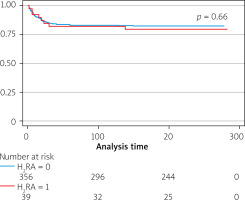
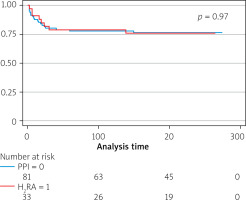
The study period was 6.3 ±2.9 months. During this period 71 patients died – 8 (20.5%) were taking H2RA. There was no significant difference in mortality between H2RA users and non-users (HR = 0.84, 95% CI: 0.35–2.05) and the PPI group (HR = 0.34–3.19, 95% CI: 0.34–3.19). The use of H2R did not impact the readmission (HR = 1.43, 95% CI: 0.66–3.11) (Figures 2, 3).
Figure 3
Kaplan Meier survival curve by use of H2 receptor antagonist and proton pump inhibitor
H2RA – H2 receptor antagonist, PPI – proton pump inhibitor.
The length of stay was modestly longer in the H2RA user group as compared to the non-user group (16.08 ±15.04 days vs. 11.4 ±12.1 days, p = 0.05). The H2RA user group had similar length of stay as compared to the PPI group. The rest of the outcomes including thromboembolism, acute respiratory distress syndrome, severe COVID-19 infection, and level of care including ventilation and ICU stay were similar in the H2RA user group compared to H2RA non-user and PPI group (Tables II and III).
Table II
Independent association of the H2 blocker use compared to non H2 blocker with outcomes
Table III
Independent association of the H2 blocker use compared to proton pump inhibitors with outcomes
Discussion
Our retrospective study has demonstrated that the use of H2RA had no influence on mortality when compared to patients not on H2RA and those using PPI. The use of H2RA as a home medication was associated with modestly increased length of stay, but it had no impact on readmission rate, severity of COVID-19 infection, level of care, and thromboembolism.
Previous studies proposed that famotidine reduces the inflammation in response to SARS-CoV-2 [3]. The improved outcomes with use of famotidine in previous studies are making PPI a less attractive option for clinicians. We believe that there was selection bias that confounded the results in previous positive studies. The subjects who received famotidine were less sick and as a result had better outcomes. We were unable to produce the same result when we took patients with H2RA as a home medication into consideration.
Theoretically, the low acid levels that occur in the stomach with the use of H2RA and PPI can lower the defence against viral infections [15]. However, it is unknown if this mechanism can cause poor outcomes in COVID-19 patients. A recent study by Yeramaneni et al. [6] showed that the inpatient famotidine use in patients who were not taking this medicine at home was associated with higher 30-day mortality.
The strengths of our study are the diversity of the patient population, granularity in obtaining patient characteristics, and complete follow-up for the duration of the study period. We included patients based on H2RA or PPI as home medications, unlike published studies in which the medications were used only during the hospitalization. The risk of selection bias is minimal. Our study also has several limitations, including the retrospective study design and single-centre setting. The adjudication we tried to limit by using 2 reviewers independently collecting information from the medical records. ICD 9 and ICD 10 codes were used to identify the patients. It is possible that some patients might have been misclassified. Careful chart auditing was performed to limit the misdiagnosis. Both PPI and H2RA are utilized as over-the-counter medications, and underestimation is possible. There is variability in dosing of these medications, although the majority our patients were using a modest single daily dose.
Conclusions
The use of H2RA is not associated with improved outcomes in COVID-19 hospitalized patients, nor did it show any advantage over PPI. The clinical outcomes with use of H2RA in COVID-19 patients are inconsistent, and the management of peptic ulcer disease or stress ulcer prophylaxis management should remain unchanged during the COVID-19 pandemic. Prospective studies are required to better understand any role of acid suppressant medications in COVID-19 patients.