Inflammatory bowel disease (IBD) is a chronic condition of the gastrointestinal tract, with a complex aetiopathogenesis in which genetic, immunological, and environmental factors are being discussed. The development of knowledge about molecular processes and the aetiology of diseases are made possible the implementation of modern therapies with biological drugs, which are a heterogeneous group of substances working by modulating the body’s immune response. Currently, biological medications are used in an increasing number of indications.
We present a case of a 25-year-old female patient suffering from psoriasis from the age of 6 years, who at the time of hospitalization in the Department of Gastroenterology and Hepatology had been on a bimekizumab biological treatment program for 2 years.
Soon after starting the drug, the patient noticed slight abdominal pain and more frequent bowel movements. The symptoms became much worse over time. On admission to the hospital, the patient had severe diarrhoea with mucus and blood, lower abdominal pain, and weight loss of 5 kg over a period of 3 months.
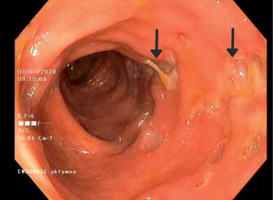
Laboratory tests showed microcytic anaemia and elevated levels of C-reactive protein and faecal calprotectin. The infectious origin of the observed symptoms was excluded. The colonoscopy revealed changes in the large intestine typical for Crohn’s disease (CD) in the active phase (Figure 1). Crohn’s disease activity index was rated at 390 points. Magnetic resonance enterography confirmed active inflammatory lesions of the large intestine. However, in the histopathological examination of the samples, the image did not meet the diagnostic criteria for CD.
The treatment included steroid therapy, as well as mesalazine and azathioprine, obtaining a good clinical and biochemical response. In view of the above, the dermatologists decided to discontinue bimekizumab treatment.
Bimekizumab is a biological drug in phase III of clinical trials, used in the treatment of psoriasis, psoriatic arthritis, and ankylosing spondylitis, which is an inhibitor of interleukin 17 isoforms A and F (IL-17 A and F).
Rare cases of IBD symptoms have been reported in the literature with other inhibitors of IL-17 such as secukinumab or ixekizumab, but none described their occurrence during the use of bimekizumab [1].
It is difficult to clearly determine whether bimekizumab causes enteritis de novo or only exacerbates the symptoms of the present disease, which has so far been asymptomatic because patients with psoriasis are more predisposed to IBD than the general population. We know, however, that attempts to use IL-17 inhibitors in the treatment of IBD have been unsuccessful due to the lack of improvement or even intensification of symptoms in some patients [2].
In conclusion, the increasing use of biological drugs in medicine brings many benefits, but it also allows us to observe negative side effects. The presented clinical case is not an isolated one and might justify the need to include in the ICD-10 classification a new disease entity. It is also worth determining whether these drugs cause specific diseases known to us as CD or whether they are new diseases that can be classified separately in the group of IBDs.