Introduction
Our knowledge about the development and symptoms of infection has changed dynamically since the first SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) infections were detected in 2019 in China [1, 2]. On 4 March 2020, the first case of SARS-CoV-2 infection was confirmed in Poland [3, 4]. From that time until 31 March 2022, approximately 5.96 million cases of COVID-19 (coronavirus disease 2019) were recorded in Poland [4, 5]. Initially, the disease was mainly related to the respiratory system, but over time it transpired that patients with COVID-19 also have gastrointestinal symptoms [6–8].
In the context of inflammatory bowel diseases (IBD), which include Crohn’s disease and ulcerative colitis, numerous questions arose from patients and became new challenges for gastroenterologists worldwide. On the one hand, the predisposition to inadequate immune response and the immunosuppressive treatment used make patients with IBD a group of people with an increased risk of infections, which may be atypical and resistant to treatment. On the other hand, several doubts have arisen regarding the potential impact of infection on the course of IBD [9–11]. Recently, much research has been devoted to this issue, but most of it originates from the countries of Asia, Western Europe, and the United States [12–23]. Bearing in mind, i.a., the possible impact of geographic location, eating habits, the incidence of SARS-CoV-2 infection, differences in the availability of certain drugs, and many other elements individually characterizing particular populations, we decided to expand this knowledge based on a single-centre analysis of the course of COVID-19 infection in IBD patients treated in the Greater Poland voivodship in Poland.
Aim
The main objectives of the study were to assess the frequency of SARS-CoV-2 infection in the group of patients diagnosed with IBD, the potential impact of IBD and IBD treatment on the course of COVID-19, and the clinical manifestations of COVID-19 infection in this group of patients. The analysis was carried out before the implementation of protective vaccinations against SARS-CoV-2.
Material and methods
We performed a retrospective analysis of data on SARS-CoV-2 infection among IBD patients hospitalized at the Department of Gastroenterology of Poznan University of Medical Sciences from 1 March 2020, to 31 May 2021, due to underlying gastrointestinal disease with a positive history of SARS-CoV-2 infection. The analysis included only patients with infection confirmed by PCR or antigen test based on a nasopharyngeal swab, following the updated guidelines for diagnosing SARS-CoV-2 infection in Poland [3]. The data characterizing the study group were collected, considering the following variables:
age, gender,
IBD type (Crohn’s disease vs. ulcerative colitis),
body mass index (BMI),
comorbidities,
IBD activity at the time of COVID-19 diagnosis (assessed qualitatively as remission or active disease, and additionally semi-quantitatively as a subjective assessment by a physician according to a 4-stage gradation, differentiating remission, mild, moderate, and severe relapse),
treatment: 5-ASA (mesalazine), corticosteroids, thiopurines, biological treatment at the time of COVID-19 diagnosis,
new gastrointestinal symptoms that developed during COVID-19,
duration of symptomatic COVID-19,
the need for hospitalization for COVID-19, including hospitalization in an intensive care unit,
death due to COVID-19.
The criterion of severe COVID-19 disease was illness requiring hospitalization, including hospitalization in an intensive care unit or death due to COVID-19.
Statistical analysis
Continuous data were presented as means and standard deviations or medians with interquartile ranges (IQR) or 95% confidence intervals (CI). The hypotheses that data follow a normal distribution were checked by Shapiro-Wilk test.
Differences in the analysed factors between the groups distinguished according to the course and the need for hospitalization in COVID-19 disease were detected by Student’s t-test or the Mann-Whitney U-test for data with or without normal distribution, respectively. Categorical data were presented as percentages, and differences between groups were detected by Fisher’s exact tests.
The associations between the duration of COVID-19 symptoms and other analysed factors were evaluated using Spearman’s rank correlation coefficient. Mann-Whitney U-tests or Kruskal-Wallis tests were used to examine the significance of differences in the duration of COVID-19 symptoms among groups.
A p-value below 0.05 (2-sided) was considered statistically significant. All analyses were performed with the statistical package Statistica v. 13.1 (Stat Soft. Inc., Tulsa, OK, USA).
The study did not require the approval of the bioethics committee due to its retrospective nature.
Results
Among 350 patients hospitalized in the analysed period, SARS-CoV-2 infection was diagnosed in 32 (9%) patients (14 with ulcerative colitis, 18 with Crohn’s disease). SARS-CoV-2 infection was confirmed by real-time PCR in 29 (90%) patients and by rapid antigen test in 3 (1%) cases.
Severe course of COVID-19 was reported in 6 (19%) patients, but none of the patients required hospitalization in the ward of the intensive care unit, including ventilator treatment, and no cases of death due to COVID-19 were reported. Most of the patients (26/32; 81%) had a non-severe course of COVID-19. The differences in the clinical profile, treatment, and gastroenterological symptoms caused by SARS-CoV-2 infection between IBD patients with severe and non-severe COVID-19 are presented in Table I.
Table I
Comparison of clinical presentation of patients with inflammatory bowel diseases and COVID-19, who required hospitalization (severe COVID-19), with those who did not require hospitalization due to infectious disease (non-severe COVID-19)
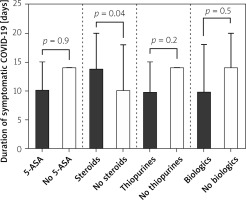
IBD patients treated with systemic steroids were more likely to have significantly (p = 0.04) higher durations (14 days; 95% CI: 10–20 days) of symptomatic COVID-19 when compared with those without steroids (10 days; 95% CI: 0–18 days). Symptomatic COVID-19 duration was significantly shorter among IBD patients on thiopurines (10 days; 95% CI: 0–15 vs. 14 days; 95% CI: 10–14; p = 0.02). No differences were detected when IBD patients were compared in regard to 5-ASA treatment (on 5-ASA: 10 days; 95% CI: 10–15 vs. without 5-ASA: 14 days; 95% CI: 10–14; p = 0.9) or biologic treatment (on biologics: 10 days; 95% CI: 10–18 vs. 14 days; 95% CI: 10–20; p = 0.5). These data are presented in Figure 1.
The relationships between other selected elements of the clinical characteristics of IBD, IBD treatment, and the duration of symptomatic SARS-CoV-2 infection are presented in Tables II and III.
Table II
Duration of symptomatic COVID-19 according to selected characteristics of the study group
Table III
Correlations between the duration of symptomatic COVID-19 and selected variables characterizing patients with inflammatory bowel diseases
Discussion
It is well known that the aetiology of IBD is unclear, and it is difficult to point out a single factor responsible for the development of the disease [20, 21]. Multiple theories focus on an abnormal immune system response to various antigens as the cause of IBD that triggers an uncontrolled inflammatory activation with cytokine release. This phenomenon, and the immunosuppressive treatment frequently used in Crohn’s disease and ulcerative colitis, make IBD patients a group of people at increased risk of infections [22]. Considering that during the SARS-CoV-2 infection a cytokine storm may also occur, the course of COVID-19 can be unpredictable in an immunocompromised host. However, the data on the associations between SARS-CoV-2 infection and IBD, originating from different countries, are not homogenous [10–18, 23]. Several individual factors, specific for particular populations, can play a significant role in determining the interplay between COVID-19 and immune-mediated disorders like IBD. That is why, although the dynamic development of the COVID-19 pandemic has obligated gastroenterologists worldwide to increase their general knowledge about the impact of SARS-CoV-2 on the gastrointestinal tract, local and national characteristics of the studied populations should also be taken into account [12–18, 23].
Initially, it was expected that the incidence of SARS-CoV-2 infections would be higher among IBD patients [11]. However, the conclusions from many international analyses have not confirmed this hypothesis [10, 14]. Based on the results of patients hospitalized in our centre, the frequency of COVID-19 infection was approximately 9% and was slightly higher than the incidence of infection in the general Polish population (7.5%; 2,872,283/38,151,000 people) in the analysed period [4, 5]. On the one hand, the relatively high risk of COVID-19 may result from the general characteristics of IBD patients, often malnourished and with an impairment of the immune system’s functioning as a result of immunosuppressive therapy and immune-mediated disease by itself. On the other hand, it should be borne in mind that the numbers were calculated based on data from patients with the most severe IBD forms who required hospital treatment. The COVID-19 incidence would probably have been lower in our cohort if the study had included patients with IBD who were successfully treated in an ambulatory setting. The same conclusions were obtained in studies on similar subjects, but they were related to the patient populations of Western Europe, the United States, and India [10–12, 14, 17]. That is why, based on the analysis, we can assume that the risk of SARS-CoV-2 infection among patients with IBD in Poland does not differ significantly from that of patients from other regions of the world.
Another critical question refers to the risk factors of severe COVID-19 among IBD patients. Which factors are important, and are there any differences in this respect considering the geographical origin of the analysis? In the general population it was shown that age over 65 years, male gender, obesity, and comorbidities (chronic respiratory and cardiovascular diseases, type 2 diabetes, liver failure, cancer, past stroke) predispose to severe COVID-19 [10, 14, 24–27]. In parallel, there is a growing body of evidence showing that also some more disease-specific factors can increase the probability of poor prognosis among SARS-CoV-2-infected individuals [13].
In one of the most comprehensive, multicentre studies from Italy, individual factors were analysed in terms of the risk of severe COVID-19 in IBD patients, such as country of residence, age, gender, IBD type, disease activity (Mayo score > 2 for ulcerative colitis and Harvey-Bradshaw Index > 4 for Crohn’s disease), current treatment, nicotinism, and comorbidities [10]. Hospitalization was required in 24% of patients, treatment in the intensive care unit in 4% of cases, pneumonia was diagnosed in 22%, and death in 1% of patients. The authors found age over 65 years and treatment with corticosteroids to be associated with the need for hospitalization and an increased risk of pneumonia. At the same time, treatment with monoclonal antibodies had a protective effect on the risk of severe disease.
Similar conclusions were drawn from the SECURE-IBD initiative, in which more than 6000 IBD patients (originating mainly from the United States) infected with SARS-CoV-2 were analysed. It was shown that systemic steroids and treatment with thiopurines increased the probability of a complicated COVID-19 course, whereas biologics seemed to have a protective effect. In one of the most recent sub-analyses, the authors also suggest that active disease might constitute an independent risk factor of hospitalization and/or death due to COVID-19, especially in younger IBD patients [25].
In addition to that, Singh et al. in a multicentre research study were able to point out several comorbidities that might predispose to the severe course of COVID-19, including poorly controlled arterial hypertension, chronic lung disease, diabetes, ischaemic heart disease, chronic kidney damage, heart failure, cerebrovascular disease, nicotine addiction, and alcohol abuse [14].
The results of our study seem to be in accordance with current data coming from other populations. We showed that patients with active, uncontrolled disease using systemic steroids were at higher risk of severe COVID-19. Moreover, the duration of symptomatic infectious disease correlated also with steroid treatment, as well as with the number of concurrent comorbidities. Older age also tended to be associated with the increasing number of days of COVID-19 symptoms. At the same time, the duration of infectious disease was lower among patients on thiopurines, and biological treatment seemed to be related to a decreased risk of severe COVID-19 (borderline significance).
Several factors must be taken into account when interpreting these results. The oldest patient in our cohort was 55 years old, so we could not show that age influenced the risk of severe COVID-19 probably due to a lack of elderly patients in our group. Nevertheless, even considering this limitation, we were able to show that increasing age must be considered when analysing IBD patients’ profiles in the context of possible poor outcomes of SARS-CoV-2 infection.
Another aspect worth mentioning is the hypothetical association between IBD-specific treatment and the clinical course of COVID-19. According to the current knowledge and studies published so far, our data confirmed that systemic steroids should be considered a risk factor for complications in SARS-CoV-2-infected individuals, whereas biologics might have a protective effect [26–28]. Data on the influence of thiopurines are more heterogeneous. As discussed above, results from the SECURE-IBD cohort suggest that azathioprine and 6-mercaptopurine might increase the risk of severe COVID-19. At the same time, a meta-analysis by Alrashed et al. did not confirm any relationship between thiopurine monotherapy and unfavourable outcomes of SARS-CoV-2 infection [28]. Nevertheless, in all these analyses it is difficult to clearly show whether the particular drug is independently influencing the course of COVID-19 or whether this association is more complex. It cannot be excluded that the ability to control the disease activity and induce remission due to a specific therapy are more important. One can hypothesize that effective treatment by using biologics or thiopurines can protect patients from complications after SARS-CoV-2 infection.
In our study, we were also able to demonstrate a general positive correlation between the number of comorbidities and the duration of COVID-19 infection. However, the number of cases with comorbidities was too small to precisely define which diseases were the most common and which could predispose patients more to prolonged symptomatic infection. However, it should be mentioned that the most frequently reported comorbidities were cardiovascular and pulmonary disorders (data not shown), which is in accordance with our previous knowledge in this respect [10, 14].
Finally, it should be stressed that the possible discrepancies in identifying risk factors of severe COVID-19 in different studies can be strictly related to the exact definition of this condition. It is worth noting that such a definition, which the whole scientific community would commonly accept, does not exist. The predefined criteria of severe COVID-19 adopted in the present study were the necessity of hospitalization and/or death due to infectious disease. We showed that 18.7% of IBD patients in our cohort met these criteria. Additionally, none of the patients required treatment in the intensive care unit, and there were no cases of death due to COVID-19. One of the studies adopted a similar definition as in our analysis, including an additional one: mortality within 30 days of COVID-19 diagnosis [13]. The clinical outcomes of IBD patients compared to the general population were similar, and no differences were found in the risk of severe COVID-19 between patients with IBD and without IBD. Other criteria of severe COVID-19 used by the scientific community were the need for treatment in an intensive care unit and SARS-CoV-2 pneumonia [10, 14, 28]. It seems, however, that the need for hospitalization due to COVID-19 encompasses most of them. Considering these details, it seems that the prognosis of IBD patients infected with SARS-CoV-2 is good and does not differ from the general population. Moreover, our data do not show any additional risk for Polish IBD patients compared with populations originating from other countries [10, 13].
Another important issue that needs to be discussed is the possible influence of SARS-CoV-2 infection on new intestinal symptoms in IBD patients. Diarrhoea, nausea, and abdominal pain were the most frequently reported new abdominal symptoms in our cohort. Moreover, these manifestations were numerically more prevalent in patients fulfilling the criteria of severe COVID-19. These data are in accordance with previously published studies. For example, the SECURE-IBD analysis showed that diarrhoea was the most common intestinal symptom in IBD and COVID-19 patients [29]. Another analysis also confirmed that the most frequent symptoms related to SARS-CoV-2 infection in the IBD population were nausea (and vomiting), abdominal pain, diarrhoea, and – additionally – loss of appetite [14]. At the same time, the authors showed that all these manifestations were significantly more prevalent in IBD when compared with controls without any inflammatory intestinal disorder.
From a pathophysiological point of view, several hypotheses try to explain these phenomena. Neurath suggested that this is probably related to the increased expression of the ACE2 (angiotensin-converting enzyme 2) receptor in enterocytes of the small intestine and colon, which binds the SARS-CoV-2 and allows it to enter cells [24]. Among IBD patients, a higher expression of this receptor was shown, especially in patients with ulcerative colitis. Hypothetically, this mechanism could explain the appearance of new intestinal symptoms in IBD patients with SARS-CoV-2 infection and might also be associated with the exacerbation of the existing inflammation in the intestines.
Like all scientific analyses, our study has limitations, some of which are discussed above. Additionally, a possible limitation of our analysis could be the time in which it was performed. During this period, vaccinations in Poland were recommended among older patients, and none of our patients was included in the COVID-19 vaccination program for a given age group. On the other hand, this is the first paper describing the associations between the characteristics of Polish IBD patients and the course of SARS-CoV-2 infection. A multicentre national study should be conducted to analyse this issue more thoroughly, in which also vaccinated IBD patients would be included. This kind of comparison would allow us to define all the benefits resulting from the anti-SARS-CoV-2 vaccination program in the Polish IBD population.
Conclusions
The prognosis in patients with IBD treated in our centre was good. All patients survived COVID-19 without short-term complications. Our study confirms for the first time that Polish IBD patients share similar risk factors of severe COVID-19 as patients in Western Europe or the U.S. After the diagnosis of COVID-19 in patients with IBD, previously initiated biological treatment and treatment with thiopurines should not be discontinued. It seems that the most important risk factor of severe COVID-19 in IBD patients is an uncontrolled, active disease and the need for systemic treatment with steroids. Adherence to general recommendations regarding viral infections, such as avoiding crowded places, disinfecting hands, and contacting a healthcare professional in the early stages of viral symptoms may be an additional protective factor. Patients with IBD should be informed about the risk of SARS-CoV-2 infection when treated with corticosteroids, as well as the need for careful compliance with protective measures.