Gastrointestinal stromal tumours (GIST) are rare neoplasms arising from the interstitial cells of Cajal [1]. The prevalence of GIST is reported to be 0.70 per 100,000 individuals per year, and up to 30% of cases demonstrate malignant potential [2]. The jejunum is a rare location for these neoplasms and, in comparison to gastric GISTs, is considered to have a less favourable prognosis [3]. Generally, small bowel bleeding is the cause of 5–10% of gastrointestinal bleeds and is often a diagnostic challenge for GI practitioners. We present a case of a jejunal GIST manifested as recurrent gastrointestinal bleeding, and an overview of an evidence-based, small bowel bleeding diagnostic strategy.
A 65-year-old male was admitted to our hospital for further investigation of recurrent, massive melena, which he had experienced twice in the previous 6 months. In both of those previous episodes, he reported painless, dark-coloured stool following a syncopal episode with a haemoglobin decrease to 7 g/dl requiring a blood transfusion. The patient had undergone outpatient esophagogastroduodenoscopy (EGD) and colonoscopy twice, which failed to identify the source of bleeding. Therefore, he was admitted to our department for further evaluation. The patient denied any history of nonsteroidal anti-inflammatory drug use, peptic ulcer, or chronic liver disease. The patient’s comorbidities included hypertension, laparoscopic cholecystectomy 7 years prior due to cholelithiasis, and subsequent hepaticojejunostomy due to choledocholithiasis 3 months later. The physical examination upon admission was unremarkable. Laboratory analysis showed microcytic anaemia (Hgb 9.2 g/dl, MCV 77 fl, Fe 6 µmol/l), while other biochemical parameters were within normal limits. Transabdominal ultrasound and chest X-ray were unremarkable.
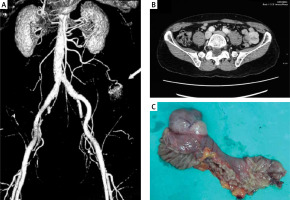
EGD and colonoscopy performed within the first 24 h of patient presentation were both negative. There was no recurrent GI bleeding during the hospitalization. Repeat EGD also showed no abnormalities except for Helicobacter pylori-negative gastritis. Repeat colonoscopy with terminal ileoscopy revealed multiple (up to 10) small arterio-venous malformations in the left colon without signs of bleeding. After assuming the bleeding originated from the small bowel, we performed multi-slice computed tomography (CT) angiography, which identified a 3 cm × 3 cm small bowel tumour mass with a vascular supply originating from the superior mesenteric artery (Figures 1 A, B). Multislice CT enterography demonstrated a well-defined tumour mass arising from the jejunum, suggestive of GIST. The patient underwent laparoscopic surgical resection of the ulcerated mass lesion. There was no evidence of mesenteric lymphadenopathy (Figure 1 C). The histology of the resected specimen was consistent with a low-grade GIST with a TNM staging of T2, N0, M0. Immunohistochemistry (IHC) was positive for CD117, CD34, and actin SMA-α and negative for desmin and S-100 protein. After 2 years of clinical follow-up, the patient remains symptom-free with no evidence of recurrence.
Figure 1
MSCT angiography, coronal view, arterial phase showing tumour mass 3 cm × 3 cm supplied from the superior mesenteric artery (A); axial view, venous phase (B); resected specimen of jejunal GIST measuring 3 cm × 3 cm × 3 cm (C)
This case describes the clinical presentation of a rare jejunal tumour. Because our patient had prior hepaticojejunal anastomosis and colonic arterio-venous malformations, it was first suspected that the GI bleeding arose from one of these sources. The clinical presentation of GIST is usually nonspecific, found incidentally, and varies due to its anatomic location and size [4–6]. However, they may become symptomatic as a result of complications such as bleeding, intussusception, or bowel obstruction. GISTs may manifest with occult and/or overt obscure GI bleeding, as in our case, although occult bleeding is more common. Furthermore, bleeding from GISTs has been shown to be a poor prognostic sign [3].
Despite the advances that have been made in the field of small bowel imaging such as the introduction of video capsule endoscopy (VCE), balloon-assisted enteroscopy, and now motorized spiral enteroscopy, the diagnosis and management of small bowel bleeding (SBB) remain challenging. Guidelines on SBB published by the American Gastroenterology Association (AGA), the European Society of Gastrointestinal Endoscopy (ESGE), and the Japanese Gastroenterological Endoscopic Society (JGES) vary somewhat on recommended management strategies [7–9]. One of the key differences between the guidelines is that CT is placed higher in the algorithm proposed by the Japanese society [9]. In our case, performing CT angiography was the key to a successful diagnosis.
The differential diagnosis of small-bowel bleeding should include the most common causes, such as arterio-venous malformations, tumours, and nonsteroidal anti-inflammatory drug (NSAID)-induced enteropathy [10]. Also, rare entities should be considered such as Meckel’s diverticulum, Dieulafoy’s lesions, small-bowel varices, diverticula, and aortoenteric fistula [11, 12]. The bleeding aetiology may vary depending on the age of the patient. Patients older than 50 years are more prone to bleeding from vascular lesions, while small bowel tumours are more common in patients under the age of 50 years [13].
The initial evaluation of SBB should include a detailed history and physical examination, followed by appropriate diagnostic evaluations. In cases of high suspicion of small bowel bleeding, as was in our case after 2 negative EGDs and colonoscopies, the next appropriate diagnostic step depends on several factors, one of which is the assessment of the patient’s haemodynamic status. If the patient is haemodynamically unstable, computed tomography with angiography (CTA) should be the next step, with high accuracy (90%) in the detection of acute GI bleeding [14]. Standard angiography has its limitations, one of which is the rate of bleeding, which must be ≥ 0.5 ml/min for active bleeding to be detected [15]. If GIST is encountered at endoscopy, its typical feature is a well-delineated ball-like mass arising subepithelially [16]. Endoscopy may be followed by endoscopic ultrasound (EUS), if anatomically accessible, to assess the extraluminal extent of the mass. In cases of malignant GISTs, features suggestive of malignancy on EUS include irregular margins, lobularity, and enlarged lymph nodes [17].
The treatment of GIST depends on several factors. Before any therapeutic intervention, evaluation with EUS or CT must be performed for disease staging. If found at an early stage without metastasis, endoscopic resection provides a minimally invasive, effective, and safe treatment. In cases of small (< 20 mm) GISTs, endoscopic resection by means of endoscopic full-thickness resection (EFTR) may be appropriate. Guo et al. reported a 100% complete resection rate with EFTR in a case series of 23 gastric GIST lesions [18]. He et al. reported in a cohort of 31 patients that endoscopic submucosal dissection (ESD) was feasible and safe even in large oesophageal and gastric GISTs (measuring 2–5 cm) [19]. Because the tumour in our case was in the distal jejunum, we decided to refer the patient for surgery. The definitive treatment for GISTs remains surgery, whereas in cases of unresectable or metastatic disease, imatinib is the first-line therapy [20].
This case highlights the importance of considering small bowel gastrointestinal stromal tumours as the cause of obscure overt massive GI bleeding, and it favours CTA as the method of choice in achieving the correct diagnosis.










