Introduction
α-Fetoprotein (AFP) is an oncofoetal protein with a glycoprotein structure. It is produced in the liver, yolk sac, and the gastrointestinal tract of the foetus with incomplete maturation [1]. It reaches its highest level during the first trimester and gradually begins to decline, reaching adult levels at the end of the first year after birth [2]. In clinical practice, it is elevated in hepatocellular carcinoma, yolk sac tumours, and non-cancerous liver diseases. It may also be elevated in lung, colon, pancreas, bladder, and ovarian tumours, and especially in gastric carcinomas [3–7].
Boureille first described AFP-producing gastric cancer (AFP-GC) in 1970 [8]. AFP-GC has a frequency of 1.3% to 15% of all gastric cancers [9–12]. Ishikura et al. proposed the term “hepatoid adenocarcinoma” for primary gastric carcinoma that has histological features of hepatocytic differentiation and large amounts of AFP production [13]. It is usually diagnosed at an advanced stage. Studies have found higher proliferative activity, lower apoptosis, and rich neovascularization in AFP-GC compared to AFP-negative gastric carcinomas. AFP-GC is an aggressive tumour characterized by poor prognosis due to early liver and lymph node metastasis [14, 15].
Aim
In this retrospective study, we investigated the prognostic characteristics of AFP in patients with gastric adenocarcinoma, who were diagnosed in a tertiary health-care centre in Turkey.
Material and methods
Patients
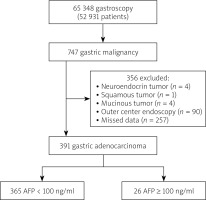
The study included patients who applied to the endoscopy unit between 1 January 2006 and 1 January 2019. Data from all 747 patients diagnosed with gastric adenocarcinoma were accessed through the hospital’s automation system. Patients with missing data in the hospital information management system and no files in the archive were excluded from the study. Thus, the study included 391 patients for whom complete data were available (Figure 1). Ethics committee approval was received from the Ethics Committee for Non-invasive Research of the hospital (date: 26/09/2019, decision number: 410).
For all patients included in the study, demographic characteristics, age, location of the lesion, age at diagnosis, date of diagnosis, levels of AFP, CEA, and CA19-9, date of operation, pathology diagnosis, results of computed tomography and positron emission tomography (PET)-CT, and survival status were recorded. Cancer staging was performed using cross-sectional imaging methods. In addition, cancer staging in patients who had undergone surgical intervention was based on post-operative findings. The cut-off value of AFP was set as 100 ng/ml. Stage III or IV was considered as “advanced stage” according to TNM classification.
Inclusion criteria:
Patients who were admitted to the Department of Gastroenterology between 2006 and 2019 and were diagnosed with gastric adenocarcinoma.
Exclusion criteria:
Patients who had undergone the first endoscopic examination at another centre;
Patients who previously had partial gastrectomy;
Patients with recurrent gastric carcinoma;
Patients with missing data;
Patients with tumours other than adenocarcinoma (neuroendocrine, squamous, mucinous);
Patients with liver disease such as hepatitis or cirrhosis;
Statistical analysis
The data were analysed using Statistical Package for Social Science (SPSS) version 22.0. First, the normal distribution of all data was assessed using the Kolmogorov-Smirnov test. Descriptive statistics are given as percentages (%). Data with normal distribution are expressed as mean and standard deviation, and those with non-normal distribution are expressed as median and minimum-maximum values. The Pearson χ2 test and Fisher’s exact test were used to compare 2 groups with categorical variables. For intergroup comparison, Student’s t-test was used for normally distributed data, while the Mann-Whitney U test was used for non-normally distributed data. An independent t-test was used to compare the numerical data of the 2 groups. Cox regression univariate and multivariate analyses were performed to determine prognostic factors in gastric adenocarcinoma.
For Cox regression analysis, CEA and CA 19-9 were calculated by converting dichotomous variables into categorical variables based on the median value. In addition, receiver operating characteristic (ROC) analysis was performed to determine the sensitivity and specificity of the AFP test. The limit of significance was set as p < 0.05 for all statistics.
Results
Our study included 391 patients who met the inclusion criteria. The mean age of the patients was 62.7 ±13.7 years. In 66 (16.9%) patients, the AFP level was higher than normal (cut-off level: 8.2 ng/dl). The demographic characteristics of the patients are shown in Table I.
Table I
Patient characteristics (n = 391)
No significant difference was found between groups with AFP levels of < 100 ng/ml and ≥ 100 ng/ml in terms of gender, location of the lesion, or stage of the tumour. An AFP value higher than 100 ng/ml was significantly associated with liver metastasis, pathological diagnoses, and metastasis status (Table II).
Table II
Comparison of patients by AFP concentration
Although univariate analysis showed that age, CEA, and CA19-9 were significant in predicting mortality (Table III), multivariate analyses revealed statistically insignificant AFP, CEA, and CA19-9 values. In addition, the localization of the gastric tumour had no effect on predicting mortality (p = 0.88).
Table III
Results of COX regression univariate analysis for predicting mortality
Cox regression multivariate analysis showed that age and an AFP level of ≥ 100 ng/ml indicate a significant increase in mortality (pAFP = 0.032 and pAge ≤ 0.001, respectively) (Table IV). Treatment parameters have been excluded in cases where it was not possible to apply standard treatment regimens due to age and comorbidities.
Table IV
Results of COX regression multivariate analysis for predicting mortality
ROC analysis showed no significant effect of AFP level on mortality. The cut-off value of AFP was set at 2.7 ng/dl, but no significantly higher level of AFP was detected (p = 0.652).
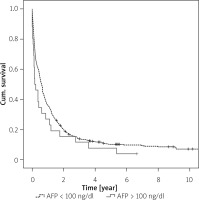
The comparison based on AFP levels (100 ng/ml) and the location of the lesion showed no significant difference in terms of time between diagnosis and mortality (Figure 2).
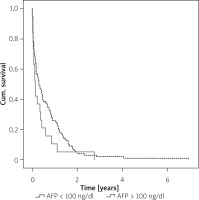
In the study, the data of patients whose AFP levels were not studied were considered as missing completely at random (MCAR) (p = 0.992) (Figure 3).
Discussion
Gastric adenocarcinoma is the sixth most common and the fourth most deadly cancer among females and males in Europe [16]. In our country, it is the third and the second leading cause of cancer-related deaths in males and females, respectively [17].
AFP is an oncofoetal antigen that is synthesized by the foetal liver, yolk sac, and gastrointestinal tract. In adults, there is a physiological elevation of AFP during pregnancy [1, 2]. Moreover, up to 25% of patients with benign liver disease have moderate elevations of AFP. AFP has a well-known role as a tumour marker in the diagnosis and follow-up of malignant hepatoma and germ cell tumours [3].
AFP-GC was first described by Boureille in 1971 as “gastric cancer with a high level of AFP” [8]. Later, some researchers defined AFP-GC as “immunohistochemical AFP positivity of tissues”, while others defined it as “AFP positivity in both serum and tissue” [15, 18, 19]. Today, it has been generally accepted that one of the diagnostic criteria for AFP-producing gastric carcinoma is AFP-positive immunostaining in the primary lesion regardless of serum AFP levels. Liu et al. showed AFP immunopositivity not only in primary lesions but also in metastatic lesions such as metastatic lymph nodes or liver lesions [15]. In our study, a serum AFP level of >100 ng/ml was considered an indicator of AFP-positive gastric cancer.
Our study included 391 patients diagnosed with gastric adenocarcinoma. Of these patients, 15.9% (n = 66) had AFP positivity. A study conducted in Europe by Webb et al. showed a rate of 15% for AFP positivity in gastric cancer, while a study conducted by Wang et al. in Japan found a rate of 30% [19, 20].
A study by Qinyi et al. showed that 62 of 82 patients with AFP-GC were male, 20 were female, and the mean age was 62.2 years [21]. Another study also showed that 70% of 328 AFP-positive gastric cancer patients were male (n = 235) [19]. In our study, 68.3% of the cases were male (n = 267), and the mean age was 62.8 years, which was consistent with the data of the literature studies.
AFP-GC is more frequently associated with lymphovascular invasion, lymph node metastasis, and liver metastasis. Therefore, it has a worse prognosis. Although its mechanism has not been fully elucidated, the aggressive properties of these tumours have been found to be associated with c-Met overexpression. The c-Met proto-oncogene is a gene responsible for cell proliferation and migration. Hepatocyte growth factor (HGF) is the ligand of this oncogene and is involved in the mobilization of epithelial cells and regeneration against liver damage. AFP increases proliferation of tumour cells through HGF and c-MET pathways [22]. In addition, AFP has a suppressive effect on lymphocyte transformation. Early vascular invasion of these tumours and the susceptibility of the liver to the growth of AFP-producing gastric cancer cells lead to high rates of liver metastasis. A study conducted by Webb et al. on 290 participants found that 15% of gastric cancer patients had elevated AFP levels, and it reported a correlation between high AFP levels and liver metastasis (p < 0.05). Wang et al. showed a rate of 60% for liver metastasis, compared to 63.4% in our study. Moreover, Webb et al. reported that multivariate Cox analysis showed no correlation between high AFP levels and survival rates, which was comparable to the results of our study (p = 0.19). Therefore, Webb et al. stated that AFP is not a valid prognostic marker for gastric cancer [19, 20].
Liu et al., in their study, divided 104 participants into 2 groups as AFP-positive (+) gastric carcinoma (study group) (cut-off value of AFP was 100 ng/ml) and AFP-negative (–) gastric carcinoma (control group). They did not find any differences between the 2 groups in terms of gender, age, curability, and localization and type of tumour, but showed a higher incidence of vascular invasion and lymph node metastasis in the study group compared to the control group (p = 0.02 and 0.02, respectively). A significantly lower survival rate was observed in the AFP-positive group compared to the stage-matched AFP-negative group (p < 0.001). Important prognostic factors of the AFP-positive group included preoperative serum CEA levels, liver metastasis, operative curability, vascular invasion, serosal invasion, lymph node metastasis, and pathological stage. Liver metastasis and pathological stage have been reported as independent prognostic factors [15]. In contrast to the study by Liu et al., our study found no significant difference in age, gender, and localization of the tumour between AFP-positive gastric carcinoma (> 100 mg/ml) and AFP-negative gastric carcinoma groups (p = 0.9, p = 0.2, and p = 0.5, respectively). This has been attributed to the presence of advanced cancer in most patients included in our study because the study centre is a tertiary hospital, consisting of patients who have applied or been referred at a late stage.
A study by Qinyi et al. that included 55 subgroups of AFP-GC showed that serum AFP levels (cut-off value was 200 ng/ml) were a prognostic factor for overall survival, with a high significance (p = 0.030). However, multivariate Cox regression analysis showed that in AFP-GC, only the TNM stage was an independent risk factor for prognosis, and AFP was associated with statistically insignificant results (p = 0.016, p = 0.43, respectively) [21]. In another study, comparison between groups with AFP levels of ≥ 300 ng/ml and < 300 ng/ml did not find a significant relationship between AFP levels and mortality [23]. In our study, AFP and age were found to be independent predictors for mortality (p = 0.032 and p < 0.0001).
Piso et al. reported that proximal gastric tumours have a more aggressive course [24]. Another study by Talamanti et al. showed similar results [25]. In our study, no significant correlation was found between the location of the lesion and the survival rate or AFP levels.
In this study, a statistically significant relationship was found between AFP levels and liver metastasis (p < 0.001). In this regard, our results were consistent with the results of the studies in the literature. In our study, patients with liver metastasis were divided into 2 groups based on the cut-off level of 100 ng/dl, and higher mortality rates were found in the group with higher AFP values, but the difference was not significant (p = 0.107).
The limitations of our study include a retrospective study design and the absence of immunohistochemical staining for AFP. Although there were a significant number of missing data in our study, they were considered as missing completely at random (MCAR) in terms of prognosis (p = 0.992), which provided bias-free statistical results.