Introduction
Today, pancreatic cancer is a clinical challenge. Annually around 200,000 new cases of pancreatic cancer are reported worldwide. This cancer is slightly more common in men (52% men vs. 48% women), and it is the 4th most common cause of mortality in both sexes. Due to the significant biological aggressiveness of the tumour itself, the lack of pathognomonic symptoms at the initial stage of the disease, as well as the lack of adequate diagnostic methods, most patients are diagnosed at a late stage [1–5].
Pancreatic cancer is more common in developed countries. Most cases are found in the United States, Western Europe, and Australia. The lowest numbers of diagnosed pancreatic cancer cases are recorded in India, Iran, and Iraq [1–3, 6–8].
In 2016, 53,000 new cases of pancreatic cancer were reported in the United States. It was found that about 3200 people in Poland suffer from pancreatic cancer annually. The annual survival rate is approximately 22.7%, and the 5-year survival rate is approximately 8.8%. The continuous increase in morbidity and hence mortality from pancreatic cancer has been deemed problematic [1, 9–12].
Incorrect nutrition or even malnutrition can accompany pancreatic cancer. Up to 80% of patients with digestive tract cancers report weight loss on admission to hospital [1, 12].
Aim
The aim of the study was to assess selected parameters of nutritional status depending on the stage of pancreatic cancer. The incidence of anaemia in this group of patients was also analysed.
Material and methods
The analysis included 93 patients treated in the II Clinical Department of General, Gastroenterological, and Oncological Surgery of the Medical University of Bialystok during the years 2008–2011. The study group included 47 women and 46 men. The mean age of patients was 62.5 years. The youngest patient was 31 years old, and the oldest patient was 87 years old. The disease severity according to TNM classification was as follows: 1st stage – 1 patient, 2nd stage – 2 patients, 3rd stage – 54 patients, 4th stage – 36 patients.
The study was approved by the Bioethics Committee of the Medical University of Bialystok and conducted according to the principles and guidelines of the declaration of Helsinki.
Results
The vast majority of patients were patients with stage III and stage IV pancreatic cancer (96.8% stage III and IV vs. 3.2% stage I and II).
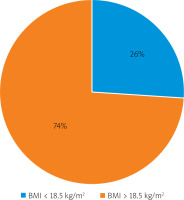
Upon admission to the department, all patients had their body mass index (BMI) calculated. In 74% of cases, normal or obese BMI parameters were seen, while 26% showed signs of malnutrition (BMI < 18.5 kg/m2) (Figure 1). There were 15 men and 9 women in the group of people with malnutrition as observed when their BMI was calculated. In 4 men, severe cachexia (BMI < 16 kg/m2) was noted, 7 patients (1 woman and 6 men) were emaciated (BMI 16–16.99 kg/m2), while BMI between 17 and 18.49 kg/m2, which represented being underweight, was found in 13 patients (8 women and 5 men) (Figure 2).
The correlation between BMI values and the stage of the cancer are as follows:
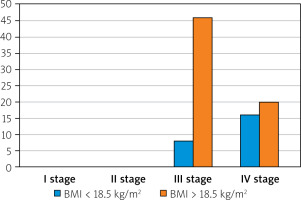
BMI values below 18.5 kg/m2 were not found in patients with stage I and stage II cancer. In the group of 54 patients with stage III cancer, only 8 patients showed malnutrition vs. 46 patients with normal BMI or even overweight.
In the group of patients with stage IV cancer malnutrition was found in 16 cases vs. 20 patients with close to normal BMI results.
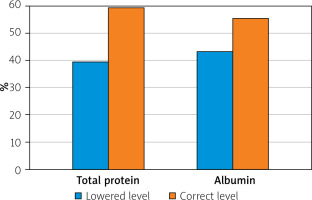
The other parameters that were assessed were total protein and serum albumin levels. Lower than normal protein levels in blood serum were found in 37 patients or 40% of the whole study group. Hypoalbuminaemia was found in 41 patients, i.e. 44% of patients (Figure 3).
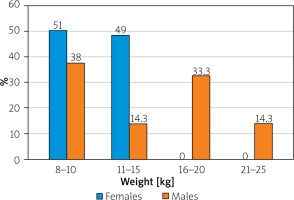
In addition to the parameters mentioned above, the degree of weight loss during the course of the disease was assessed during anamnesis. It ranged from a loss of 8–25 kg of body weight over 2–6 months. Weight loss was recorded in all 93 patients (Figure 4). In the case of 24 women (51% of all women cases in the study) a weight loss of 8–10 kg was observed (average of 9 kg), in the next 23 (49%) women it was between 11 and 15 kg (average of 13 kg). In the case of men, the weight loss observed was as follows: in 17 cases, weight loss was 8–10 kg (average of 9 kg), and in 7 patients this level was 11–15 kg (average of 14 kg). Weight loss in 15 patients was reported 16–20 kg (15.5 kg on average), while the remaining 7 patients had an average loss of 23 kg (range between 21 and 25 kg).
Anaemia was another parameter monitored among the examined patients. Both erythrocyte count and haemoglobin levels were assessed. Assessments were made during admission of the patients to the department. Anaemia of varying degrees was found in 43 men and 28 women, which was 91% of men and 60% of women, respectively. The remaining 4 men and 19 women had normal haemoglobin and erythrocyte results.
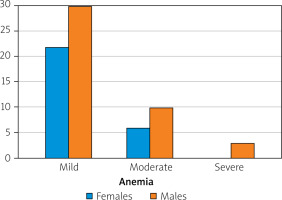
In terms of severity, patients with anaemia were divided into 3 groups: a) group 1, mild anaemia: haemoglobin concentration 10–12 g/dl, b) group 2, moderate anaemia: haemoglobin 8–9.9 g/dl, c) group 3, severe anaemia: haemoglobin 6.5–7.9 g/dl.
The first group included 22 women and 30 men. The second group consisted of 6 women and 10 men. In the last group there were only 3 men (Figure 5).
Discussion
In recent years, there have been an increasing number of publications considering pancreatic cancer and the nutritional status of patients suffering from it. The problem of malnutrition coexisting with pancreatic cancer seems to be increasing, which is associated with an increase in incidence, but also delayed diagnostics, and hence the high clinical advancement of the disease at the time of diagnosis [1, 4, 5, 12].
It is estimated that about 50% of patients with pancreatic cancer have weight loss in the initial phase of the disease, which is unnoticeable. However, when patients who have already been diagnosed are taken into consideration, this reaches 80%. In the above study, the percentage of patients with body weight loss was 100%, and it ranged from 8 to 25 kg, depending on the clinical stage of the disease. The greater weight loss was in men equal to 25 kg of total body weight loss [1, 9–12].
In most cases, initial weight loss was not noticeable, particularly in the group of women. Patients also reported slight body weight loss (1–5 kg) with changes in diet and “increased” exercise, i.e. attempts to lose weight. Similar thinking seems to have a devastating effect on the timing of diagnosis.
BMI value was another factor analysed by the authors. Body mass index seems to be the simplest tool for assessing nutritional status. Unfortunately, both in our study and in the assessment of other authors, its value seems to be debatable [1, 12].
In the studied group of 93 patients, only 24 patients (26% of the study group) had a BMI below 18.5 kg/m2, while weight loss was found in all subjects. There is still conviction among patients and doctors about the value of BMI as one of the predictive factors that indicate the severity of the disease or its possible complications. However, this is not reflected in the available literature or the authors’ research. It seems significant that higher weight loss was observed in obese patients, and even despite the loss of 15–20 kg of body weight, patients had a BMI within the normal range. This may mistakenly indicate a good metabolic condition of the patient upon admission. According to available reports, there is no statistically significant difference in the overall survival of patients with pancreatic cancer based on low or high BMI [1, 12–20].
In many papers, the problem of hypoproteinaemia and hypoalbuminaemia is raised, which seems to be inseparably connected not only with pancreatic cancer, but also with cancers of other parts of the gastrointestinal tract [1, 21–28]. We speak of hypoalbuminaemia when the serum albumin level is lower than 2.1 mg/dl. Many authors believe that this is a specific marker not only of malnutrition, but also of the severity of the disease in the case of pancreatic cancer. It was also found that hypoalbuminaemia is associated with postoperative complications such as postoperative wound infection, a higher percentage of leakage in anastomosis, or its complete failure [1, 21–24]. The albumin level also correlates with an increase in perioperative mortality. Gibbs et al. presented the relationship between perioperative mortality and albumin levels over 30 days of follow-up [22]. Albumin levels at around 4.6 mg/dl were associated with a mortality rate of around 1%. Mortality increased significantly with a decrease in albumin, reaching 28% with serum albumin below 2.8 mg/dl [1, 24]. Currently, the serum albumin level is considered as one of the factors used in prognostic scales. Unfortunately, the whole situation is complicated by the fact that albumin is a negative parameter of the acute phase, so its level decreases during the active inflammatory process (operations, injuries, infections) [1, 23–28].
The last of the examined potential prognostic factors was erythrocyte count and haemoglobin concentration. As in the case of patients with other gastrointestinal neoplasms, anaemia was observed – from moderate to severe in most patients (91% of surveyed men and 60% of surveyed women, which together constituted 76% of the study group). These results appear to be similar to those available in the literature. In addition, they suggest that the more severe the general condition of the patient and the greater severity of the disease, the worse the anaemia is [1, 12].
To sum up, in assessing the nutritional status of patients diagnosed with cancer of the gastrointestinal tract, many factors should be considered. Weight loss, albumin concentration, and total protein levels are the most important factors for assessing the severity of the underlying disease, as well as the results of treatment and predicted mortality. BMI itself is a mediocre predictor – BMI alone can mislead a patient and a doctor about nutritional status, especially if the patient was obese before the disease. Despite losing a few or even a dozen kilograms of body weight, BMI values may remain normal.
New prognostic scales are being created, taking several factors such as BMI, serum protein levels, etc. into consideration. Unfortunately, they are not specific because of the inadequacy of BMI and the function of albumin as a negative parameter of the acute phase.
Conclusions
Most pancreatic cancer patients are malnourished at the time of diagnosis.
When assessing the nutritional status of patients with pancreatic cancer, many predictive factors should be considered. Weight loss seems to be the most accurate predictor of the patient’s metabolic status, although it should never be the only parameter.
BMI seems to be the least accurate parameter for assessing nutritional status in patients diagnosed with cancer. However, when combined with weight loss and serum albumin levels, it can be quite useful as a prognostic factor.
When assessing albumin concentration, one should also remember about its function as a negative parameter of the acute phase.