Introduction
In recent decades several authors have reported that thrombocytosis was associated with higher incidence of metastases and worse prognosis in gastrointestinal tumours [1, 2]. Numerous studies have shown an association between platelets and tumours. In animal studies correlation was found between platelet inhibition [3] or depletion [4] and decreased metastasis formation. In thrombocyte-depleted animals the infusion of platelet concentrate restored the metastasising potential of tumour cells administered intravenously [5]. It is assumed that a certain mechanistic link exists between tumour cells and platelets. The thrombocytes often adhere to tumour cells [6]. On the one hand, platelets protect them from mechanical insults, and on the other hand, platelets interfere with the cell-mediated immune response: they present large amount of MHC-I antigen on their surface, and the recognition of tumour cells is compromised by the immune system because the platelets covering the tumour cells imply normal phenotype of the host [7]. Despite numerous studies, the exact pathomechanism of the relationship between thrombocytosis and tumours is not clear. Stone et al. found significant correlation not only between elevated platelet counts and thrombopoietin (TPO), but also with serum interleukin-6 (IL-6) in patients with ovarian cancer [8]. Based on those results, they suggested a possible paracrine-mediated paraneoplastic pathway: the IL-6 expressed and secreted by the ovarian tumour induces TPO synthesis in the liver, which stimulates the bone marrow and increases the platelet count. The end-result of this process is tumour-induced thrombocytosis. This pathway has not been studied in other tumours.
Aim
Our aim was to uncover any correlation between thrombocytosis and serum IL-6 in gastrointestinal tumours.
Material and methods
Between March 2015 and March 2017, 192 patients with different gastrointestinal tumours undergoing surgical intervention were evaluated in the 1st Department of Surgery of Semmelweis University. Exclusion criteria were any inflammatory disease (pneumonia, wound complication, abscess, cholecystitis, inflammation of the intravenous line, endocarditis, urological infection, Crohn’s disease, ulcerative colitis), thromboembolic complications (deep vein thrombosis, pulmonary embolism, heart attack), and corticosteroid therapy. A total of 31 patients were excluded. Platelet count and IL-6 level were defined from blood samples drawn routinely before the operation (within 4 weeks). Thrombocytosis was defined as a platelet count exceeding 400 × 103/µl. Serum IL-6 was measured with an ADVIA 2120 Hematology Analyzer.
Statistical analysis
Multivariate linear regression was applied to determine predictors of the platelet count. The coefficient and its 95% confidence interval (CI) were calculated for each variable. Pairwise associations between scale variables were analysed by Pearson correlation. All the statistical tests were two-sided, and p < 0.05 was considered statistically significant. Data were analysed by using RStudio program package (Version 1.0.143 – ©2009-2016 RStudio, Inc.).
Results
In the examined time interval patients undergoing surgery had a wide spectrum of gastrointestinal tumours regarding localisation and disease stage. We evaluated the correlation of platelet counts and serum IL-6 in each tumour type (Table I). Because the number of patients was relatively small in the subgroups, none of the correlations proved to be significant. However, a moderately strong positive correlation could be found in colon and oesophageal cancer.
Table I
Clinicopathological data
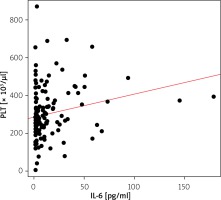
We found weak but significantly positive correlation between elevated platelet counts and serum IL-6 (correlation coefficient: R = 0.214, p = 0.006) (Figure 1).
Tumour grade did not show a significant effect on platelet counts and IL-6 level. However, if patients were divided in two groups according to disease stage – defined as early (stage 1 and 2) or advanced disease (stage 3 and 4) – IL-6 was significantly higher in the latter group (Table II).
Table II
Correlation between tumour stage and mean platelet counts or IL-6 levels
| Parameter | Early stage | Advanced stage | P-value |
|---|---|---|---|
| PLT average [× 103/µl] | 294.33 | 310.98 | 0.434 |
| IL-6 average [pg/ml] | 5.4 | 16.2 | 0.0002 |
Multivariant analysis corrected for haemoglobin, white blood cell counts, and advanced stages showed no significant correlation between IL-6 and thrombocytosis (Table III).
Table III
Correlation of platelet counts and clinical parameters with multivariant linear regression analysis
Haemoglobin showed negative, while white blood cell counts showed positive significant association with the platelet counts. A total of 12 patients had a history of thromboembolic event. We found lower platelet counts in patients with positive thromboembolic history (251 vs. 306 × 103/µl), but the difference was not significant (p = 0.153). Similarly, IL-6 was lower in this group of patients (10.3 vs. 12.8 pg/ml); however, the difference was not significant (p = 0.717). Fifteen patients took acetylsalicylic acid on a regular basis. The mean platelet count in patients on acetylsalicylic acid was 293 × 103/µl compared to 303 × 103/µl in patients without. The difference was not significant (p = 0.776). Similarly, the difference was not significant between the two groups (10.8 vs. 12.8 pg/ml) regarding the IL-6 level (p = 0.748).
Discussion
There are an increasing number of observations that chronic inflammation promotes malignant transformation of cells and tissues [9]. The strong relationship between tumour and inflammation is also supported by the fact that the use of anti-inflammatory drugs helps to prevent the development of malignant diseases.
In tumour-related inflammation tissues are infiltrated by tumour-associated macrophages (TAMs), white blood cells, and inflammatory mediators such as tumor necrosis factor (TNF), IL-1, IL-6, and chemokines (CCL2 and CXCL8), which facilitates tissue remodelling and angiogenesis [10]. IL-6 is one of the most expressed inflammatory mediators in the microenvironment of the tumour. It takes part in the regulation of almost every process of tumour growth, such as the inhibition of apoptosis [11], facilitation of cell survival [12], proliferation [13], angiogenesis [14], tumour invasion and metastasis formation [15], and metabolism of tumour cells [16]. In the microenvironment of the tumour the main source of IL-6 are the TAMs, the CD4+ T-cells, the myeloid-derived suppressor cells (MDSC), and the fibroblasts [17, 18]. In the microenvironment of the tumour IL-6 directly supports tumourigenesis with the modulation of intrinsic and extrinsic tumour cell activity [19]. IL-6 has a potential growth-stimulating effect in the tumour cells with the activation of several signalling pathways. IL-6 stimulates tumour cell proliferation and survival with the activation of Ras/Raf/MEK/MAPK, PI3K/AKT, and JAK/STAT pathways via the thyrosin phosphorylation of gp130 [13, 20]. In the inflammation transcriptional factors such as NF-κB, STAT-3 and primary inflammatory cytokines (IL-1b, IL-6 and TNF-α) are essential [21]. NF-κB is the main regulator of the inflammation that emerges from control in several tumour types. IL-6 is the main effector molecule in the NF-κB activation through the STAT-3 pathway; IL-6 is an important element of the NF-κB/IL-6/STAT-3 cascade in tumourigenesis [12]. STAT-3 is necessary to keep NF-κB activated in tumours [22], while IL-6 promotes carcinogenesis with its proinflammatory and cell-proliferative effect [23, 24]. IL-6-induced thrombocytosis is accompanied both with the mRNA expression of hepatic TPO and the increase of plasma TPO [25].
In several types of gastrointestinal cancer, elevated serum IL-6 was detected [26, 27]. STAT-3-dependent tumourigenesis correlates with the local secretion of IL-6 in colorectal cancer [12]. Additionally, IL-6 produced by M2 macrophages induces tumour development in ulcerative colitis [28]. Because the inflammation promotes gastrointestinal tumour growth and progression via the activation of IL-6-mediated STAT-3 pathway, it may be assumed that there is a strong correlation between IL-6, inflammation, and tumour progression.
Elevated serum IL-6 correlates clinically with advanced tumour stage in a variety of cancers, which implies that the inhibition of IL-6 signalling may result in therapeutic benefit. In tumours that are characterised by IL-6 overproduction, the inhibition of IL-6 signalling or the minimisation of serum IL-6 could be a therapeutic strategy.
Since the publication of Stone et al. no other study has supported or disproved their theory in other tumours. Our study does not support the paracrine-mediated paraneoplastic pathway in gastrointestinal tumours, in contrast to ovarian cancer. Although, univariant analysis showed correlation between elevated platelet counts and serum IL-6, multivariant analysis did not support this relationship. If the correlation was evaluated in the specific tumour types, a moderately strong correlation was found in colon and oesophageal cancer. Serum IL-6 was more elevated in more advanced disease, as described in the literature. The exact reason has not been elucidated yet. Thrombocytosis accompanying malignancies is often postulated as the result of anaemia. However, in our patient setting the ability of anaemia to cause reactive thrombocytosis could not be supported. Thrombocytosis showed significant correlation with white blood cell count instead of IL-6; therefore, it may be implied that inflammatory process influences both parameters.
The weakness of our study is that the study group was heterogenous and the subgroups were relatively small. Therefore, we think that a similar study on a larger group of patients is required to support our findings.