Introduction
It is generally accepted that colon cancer is the most commonly diagnosed gastrointestinal cancer and a major public health problem. As revealed by studies, the clinical outcomes of patients, especially those with lymph node-positive status, are still unsatisfactory [1, 2]. It is worth noting that approximately 20% of the diagnoses are made in the metastatic stage of the disease where the 5-year overall survival is less than 15%. However, the prognoses are not satisfactory even in the case of patients with primary tumours [3–5].
Therefore, the identification of novel approaches for describing patients from the high-risk group, particularly in the context of the clinical outcomes, has become a major challenge.
Vimentin is a 57 kDa intermediate filament protein normally expressed in myofibroblasts, chondrocytes, macrophages, and endothelial cells [6, 7]. In recent years, a great number of studies have linked intermediate filaments to signalling pathways. They can act as signalling platforms and scaffolds for different types of signalling molecules [8, 9].
As mentioned above, expression of vimentin protein has been also detected in tumour tissue, especially in tumours of the gastrointestinal tract [10]. Thus, it remains to be determined whether expression of vimentin is also a characteristic feature of colon cancer specimens and whether this expression is connected with the overall survival of Caucasian patients.
Aim
The current study investigated the expression of vimentin protein in colon adenocarcinoma samples from proximal and distal parts of the colon to assess its prognostic significance by correlating its immunohistochemical expression with the clinicopathological variables and survival of Caucasian patients.
Material and methods
Tissue samples
The study was conducted on formalin-fixed and paraffin-embedded colon adenocarcinoma samples archived in the files of the Department of Pathomorphology in Zabrze (Poland). Tissue specimens were received from 97 colon adenocarcinoma patients who underwent surgical resection in surgical clinics in 2013. The exclusion criteria were as follows: (1) history of previous malignant disease, (2) familial adenomatous polyposis, (3) inflammatory bowel disease, (4) preoperative anti-cancer treatment, and (5) evidence of distant metastasis. The clinicopathological characteristics obtained from the medical records were as follows: age, gender, location of tumour, grade of tumour differentiation, depth of invasion, lymph node metastasis, operation record, treatment record, recurrence, and vital status at the last follow-up date.
The specimens belonged to 48 men and 49 women (mean age: 68; range: 33–89 years). Tumours were located in the proximal part of the colon in 50 (51.5%) cases and in the distal part of the colon in 47 (48.45%). Three levels of differentiation were used to classify the grading as follows: well differentiated (G1), 14 (14.43%) cases; moderately differentiated (G2), 52 (53.61%) cases; and poorly differentiated (G3), 31 (31.96%) cases (Table I).
Table I
Demographic and clinical characteristics of patients included in the study (n = 97)
Immunohistochemical staining
For the immunohistochemical studies the paraffin-embedded specimens were cut into 4-µm-thick sections, fixed on Polysine slides, deparaffinised in xylene, and rehydrated through a graded series of alcohol. To retrieve the antigenicity, the tissue sections were treated twice with microwaves in a 10 mM citrate buffer (pH 6.0) for 8 min each. Subsequently, sections were incubated with rabbit polyclonal antibody to vimentin (final dilution 1 : 500) (GeneTex; cat. number GTX100619). For visualisation of protein expression, the sections were treated with a BrightVision detection system and Permanent AP Red Kit (Zytomed). Mayer’s haematoxylin was used to counterstain the nuclei.
Semi-quantitative analysis of vimentin expression in colon adenocarcinoma samples
The scores were assigned separately for the stained area and for the intensity of the immunohistochemical reaction. Quantification connected to the stained area of the tissue section was performed as follows: (1) < 33% of cells showed immunoreaction, (2) 33–66% of the cells had positive reaction to vimentin, and (3) > 66% of the cells were positive. The intensity of the immunohistochemical reaction was quantified as follows: (1) absent or weak, (2) moderate, and (3) strong. Each tissue section was characterised by a final grade derived from the multiplication of the stained area and the intensity of the staining. The vimentin expression was considered to be absent/low for grade 1; moderate for grades 2, 3, and 4; and strong for grades 6 and 9.
Survival analysis
Survival analysis was conducted in 97 patients. The survival curves were generated using the Kaplan-Meier method. The overall survival (OS) was defined as the length of time between surgery and death. The follow-up period was 60 months. Patients alive were censored at 5 years.
Statistical analysis
Statistical analyses were conducted using Statistica 9.1 (StatSoft, Poland). The clinical characteristics of the patients in relation to vimentin immunoreactivity were assessed by performing the Kruskal-Wallis test and U Mann-Whitney test. Additionally, using Spearman’s rank correlation coefficient the relationship between the immunoexpression of vimentin and age and grade of tumour differentiation was assessed. The Kaplan-Meier method was used to study survival curves, and the long-rank test to compute differences between the curves.
Results
To investigate the clinicopathological and prognostic roles of vimentin expression, the immunohistochemical analysis was performed in colon cancer tumour samples and adjacent non-pathological mucosa (Figure 1). It should be noted that only trace expression of this protein was revealed in adjacent non-tumour colon mucosa, whereas high expression was demonstrated in well-, moderately-, and poorly-differentiated tumours (Figures 1, 2). Among the 97 samples, 11 (11.34%) showed a weak immunohistochemical reaction, 35 (36.08%) demonstrated moderate immunoreactivity, and 51 (52.58%) revealed strong expression of vimentin protein.
Figure 1
Immunohistochemical expression of vimentin in colon adenocarcinoma patients according to intensity of immunohistochemical reaction
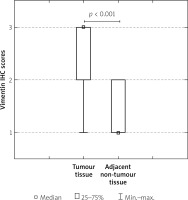
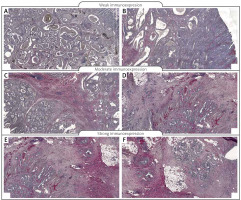
Figure 2
Immunohistochemical expression of vimentin protein in tumour tissue: A, B – weak expression of vimentin protein in colon adenocarcinoma samples, C, D – moderate expression of vimentin; E, F – strong expression of vimentin
The relationships between the vimentin levels and each clinicopathological parameter are summarised in Table II. As revealed, the level of the vimentin immunohistochemical reactivity was correlated with the grade of the histological differentiation (H [2.97] = 37.949; p < 0.001). A strong expression of vimentin protein was detected more frequently in patients with G3 tumours than in patients with G1 tumours. As revealed by the statistical analyses, a significant difference was detected between the patients with G1 tumour and G2. In the G2 patients a strong reactivity was described more frequently. The statistical evaluation of vimentin immunoexpression according to age, sex, tumour location, and depth of invasion revealed no significant difference among these variables (all p > 0.05; Table II).
Table II
Correlations between vimentin immunoexpression and clinicopathological characteristics in CRC patients
The Kaplan-Meier survival analysis showed that the overall survival rate in the group of patients with a low vimentin immunoreactivity was significantly longer than that for patients with a moderate or strong level of vimentin protein expression (p < 0.001; Figure 3).
Figure 3
Kaplan-Meier survival curves of colon adenocarcinoma patients with weak, moderate, and strong expression of vimentin protein; follow- up period = 60 months
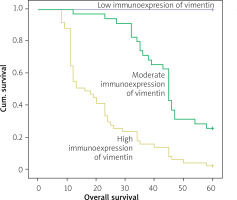
The 5-year overall survival for patients with a low, moderate, or strong level of vimentin immunoexpression was 100%, 25.7%, and 2%, respectively. The vimentin-moderate patients had an average survival time of 44.886 months (95% CI: 40.855–48.916), whereas the vimentin-strong expression groups had an average survival time of 21.706 months (95% CI: 17.863–25.549)(Table III).
Table III
Means and medians for survival time of CRC patients according to GRP94 immunoexpression
In the univariate analysis the grade of tumour differentiation and vimentin immunoexpression were found to be significantly associated with reduced 5-year survival. However, a multivariate analysis demonstrated that grade of tumour differentiation (HR = 2.150; 95% CI: 1.380–3.349, p = 0.001) and vimentin expression (HR = 3.901; 95% CI: 2.436–6.247, p < 0.001) were independent risk factors for worse survival (Table IV).
Table IV
Multivariate analyses of various prognostic parameters in CRC patients using Cox regression analyses
Discussion
The results of our study demonstrate that, in comparison to control samples of non-tumour colon mucosa, the samples of colon cancer were characterized by a high level of vimentin immunoreactivity. Moreover, the high level of vimentin immunoexpression was demonstrated to be associated with malignancy-related clinicopathological factors and 5-year overall survival of Caucasian patients. The high immunoexpression of vimentin was significantly related to advanced histological tumour grade. The Kruskal-Wallis test showed a statistically difference between the patients with G1 tumours and those with G2 tumours, and between the patients with G1 tumours and those with G3 tumours. In both cases, patients with G1 tumours had a low level of vimentin immunoexpression (p < 0.001). Similar results have been described in patients with gastric cancer. For example, vimentin expression was observed in patients with advanced stage of cancer, especially in the group with a macroscopically scirrhous type of gastric carcinoma. In other cases, expression of this protein was closely related to the diffuse type of disease, lymphatic invasion, and lymph node metastasis [11–15]. Vimentin expression was associated with significantly higher incidence of lymph-node metastasis also in the case of oesophageal squamous cell carcinoma (ESCC). The ESCC patients characterised by high expression of vimentin had significantly worse prognosis, which relates to advanced tumour status and lymphatic invasion [16–18]. The overall survival in the group characterised by high level of vimentin immunoreactivity was about 2%, in comparison to 100% in the group with low expression [19]. In pancreatic ductal adenocarcinoma (PDAC) vimentin expression is correlated with poor histological differentiation [20]. Hong et al. revealed that pancreatic cancer tissue had a threefold higher level of vimentin expression than colon, lung, and ovarian tumours. Interestingly, a more specific antigenic form of vimentin (MW 53.3 kDapI 5.1) was expressed at a 5–10 higher level in pancreatic tumours in comparison to the tumours of lung or colon. Furthermore, this expression was approximately 50% higher than that observed in pancreas without any pathological changes [21]. In HCC patients the level of E-cadherin and vimentin expression also predicted survival. In this case, the vimentin expression level was clearly correlated with shorter disease-free survival (DFS) and overall survival (OS). Moreover, statistical analyses demonstrated also the correlations between vimentin expression and poor tumour differentiation, vascular invasion, or extrahepatic recurrence following curative surgery [22, 23].
As revealed by Ngan et al. in the case of colon cancer, the prognostic power of vimentin expression (risk ratio = 3.5) seems to be better than that of lymph node metastasis (risk ratio = 2.2) [24].
The increased level of vimentin immunoreactivity especially in the stromal compartment of the tumour may indicate dynamic changes during tumour progression, e.g. fibroblastic changes, appearance of new vessels, and appearance of lymphocytes – tumour-infiltrating lymphocytes (TIL) [22]. Vimentin plays a crucial role in the development and progression of gastrointestinal cancers but also serves as a potential diagnostic marker to predict patient survival. Expression of vimentin is mainly characterised during the EMT process, and it seems that some events in the metastatic process such as cell migration and invasion are consequences of vimentin overexpression in tumour cells. However, it should be noted that in some cancers, e.g. colon cancer, vimentin expression is detected more frequently in stromal cells. Nevertheless, also in this case the presence of vimentin predicted survival of patients and was connected with worse prognosis.










